First Messenger RNA, RSV Vaccine Patient Dosed in Jacksonville, Florida
First Messenger RNA, RSV Vaccine Patient Dosed in Jacksonville, Florida
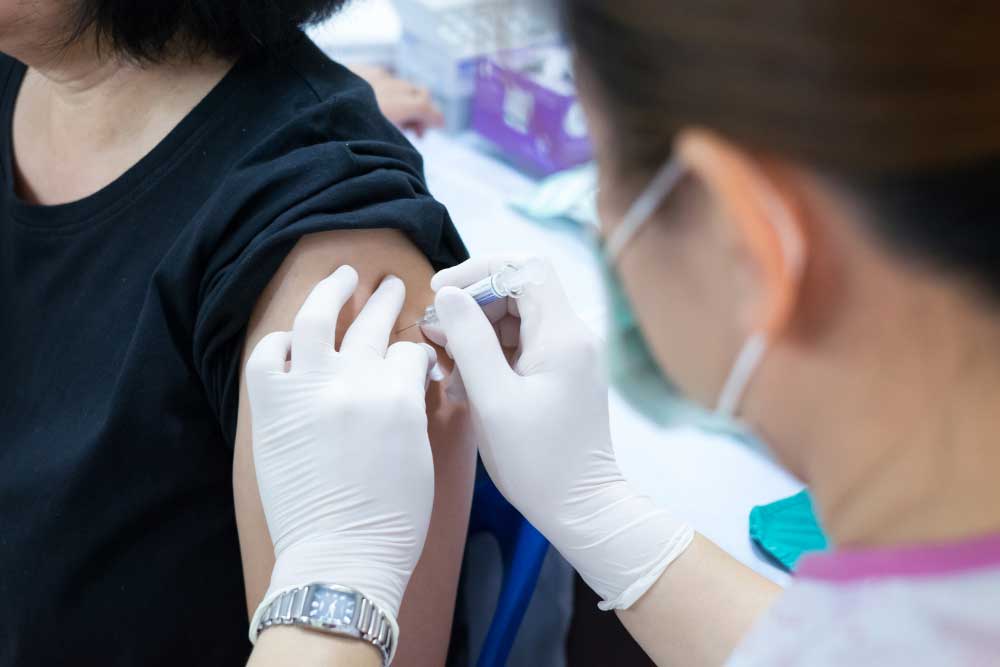
The first patient in a phase 2 study in the United States received a new, investigational Respiratory Syncytial Virus (RSV) vaccine in Jacksonville Center for Clinical Research.
11/17/2021- Jacksonville, Fl – Jacksonville Center for Clinical Research, a member of ENCORE Research Group, became the first in the United States to dose a new, investigational, Messenger RNA (mRNA), RSV vaccine to a patient in a phase 2 trial sponsored by Moderna, Inc. This helps the U.S. become closer to finding a preventative vaccine for RSV. RSV commonly has symptoms similar to influenza, COVID, and other highly pathogenic viruses.
“It is exciting to be a part of history,” Amber DeVries, study coordinator for the trial, says. “More than anything, though, it is an important step toward finding a vaccine for a virus that can be deadly.”
RSV infection rate has been on the rise, with cases skyrocketing in the summer of 2021. The cases were increasing so rapidly that the CDC issued a Health Alert on June 10, 2021. RSV is a virus that mainly affects children and adults over 65. RSV may cause severe complications to immunocompromised people.
“We should talk about and protect ourselves from the dangers of RSV,” Dr. Michael Koren, CEO of ENCORE Research Group, says. “COVID has captured our focus and rightfully so, but RSV cases have risen and continue to increase. Teamwork from the ENCORE Research Group, Moderna, and the clinical trial participants, has brought us one step closer to eradicating this virus.”
The RSV vaccine uses the same mRNA technology as the breakthrough COVID vaccines. The mRNA technology provides real-time instructions to make the proteins necessary to help our immune system fight off RSV. Many physicians and scientists believe mRNA has significant potential to improve patients’ lives.
ENCORE Research Group is a premier clinical research organization with multiple research offices in Florida; three are located in Jacksonville. ENCORE Research Group sites have conducted more than 2,500 clinical trials over 24 years and have worldwide recognition for providing patients access to cutting-edge medical research.
People interested in participating in a clinical research trial can visit encoredocs.com or call our headquarters office at (904) 730-0166
Contact: Sharon Smith
Phone: 904-730-1066
Email: ssmith@encoredocs.com

