-
Novo Nordisk’s Wegovy (semaglutide) for weight loss
-
Biogen’s Aduhelm (aducanumab) for Alzheimer’s Disease
-
Pfizer’s PREVNAR 20 (pneumococcal 20-valent conjugate vaccine) for the prevention of pneumonia
GRID VIEW
https://www.nejm.org/doi/full/10.1056/nejmoa2102214
Novavax vaccine – Among a subgroup of HIV-negative participants, the vaccine was 60.1% efficacy against the B.1.351 South African variant.
You may have heard that people with diabetes are at a higher risk of contracting COVID-19. This is not the case. The truth is, people with diabetes are more likely to experience severe illness, long lasting effects, or even death if they are infected with COVID-19.
What We Know about Diabetes and COVID-19
In May, a nationwide multicentre observational study called the CORONADO study, observed the mortality risk in people with diabetes who were hospitalized for COVID-19. The study population was 88% type 2 diabetics and 12% type 1 diabetics. What they found was that one in ten diabetic patients hospitalized with COVID-19 died within seven days of hospital admission. One in five died within the first 28 days.
How Can We Improve These Numbers?
- Metformin – Recent studies have shown that metformin decreased the mortality rate of diabetic patients with COVID-19. Those who took metformin had an 11% mortality rate compared to 24% with type 2 diabetes who were not taking metformin when admitted to the hospital. These studies heavily indicate a strong, positive relationship between metformin, COVID and diabetes.
- Vaccine – another way to protect those battling diabetes from COVID-19 is to consider getting the vaccine. There have been three emergency use authorized vaccines: Pfizer, Moderna, and Johnson & Johnson. Each vaccine appears to be safe and effective in adults with diabetes. Rigorous clinical trials tested these vaccines for safety in adults of all ages, races and ethnicities and chronic health conditions.
-
-
-
-
-
- How will the vaccine affect blood sugar levels?
- Receiving the vaccine can cause symptoms of illness that can increase your glucose levels. However, if carefully monitored and correctly hydrated side effects can be minimal.
- Do diabetes medications affect the vaccine?
- Currently, there is no evidence to suggest that the COVID-19 vaccine will interact with current medications. However, it may be helpful to avoid injecting insulin or placing a glucose sensor near your vaccine injection site for several days after receiving the vaccine.
- Should I get vaccinated if I have diabetes and other health conditions?
- Complications of diabetes include heart disease and kidney disease. These conditions put one at higher risk or death from COVID-19.
- Vaccination should be a priority for patients with type 2 diabetes who are at very high risk of severe COVID-19 to help protect this vulnerable population.
- How will the vaccine affect blood sugar levels?
-
-
-
-
-
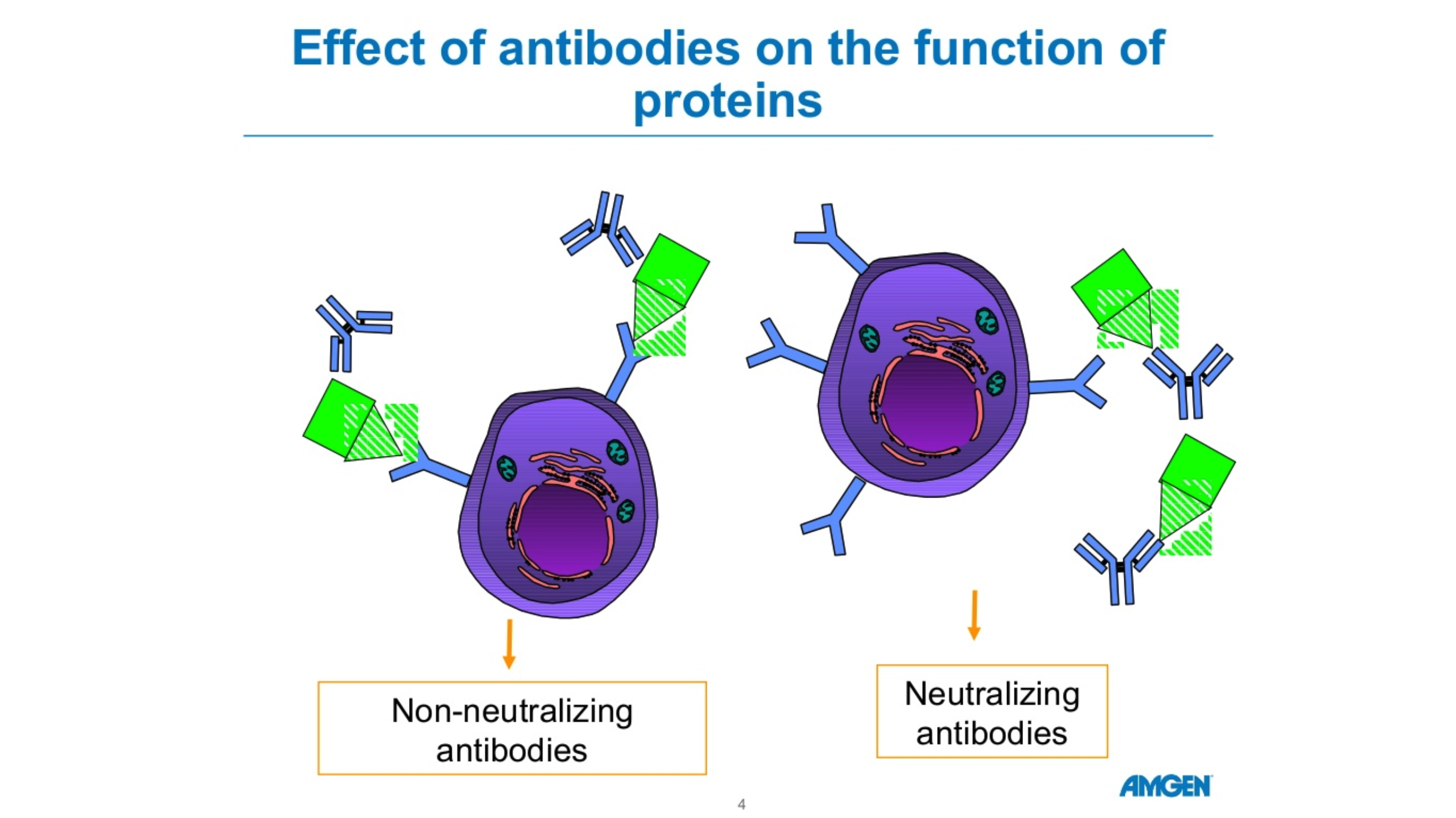
There are five forms of antibodies that the human body makes. There are two forms that are relevant for COVID 19, Igm and IgG.
Igm is a big molecule, which is the first molecule that your body makes when you are exposed to a particular antigen or virus. This is an acute phase type of antibody.
IgG is a long-term antibody that has memory for your immune system and also protects you long-term. The actual length of long-term protection is not known.
Typically, when you have antibody testing, you are tested for both Igm and IgG. These tests are not perfect. If someone tests positive for Igm but not IgG, we’re not sure if they are protected.
If someone has no Igm antibodies and lots of IgG antibodies, they’re likely protected due to the long-term memory of IgG.
The length of time the antibodies remain detectable following an infection is not known.
Source:
cdc.gov
Amgen Powerpoint
Our mission at ENCORE Research includes educating our community about health care news, particularly when standard media sources sensationalize the news. The coronavirus or COVID-19 story falls into this category. Patients and family members are asking, “How worried should we be?”
Our simple advice, “Don’t panic, but take sensible precautions.” Recent data, often reported incompletely, support the idea that we should think about COVID-19 as a bad strain of the flu.
New viruses are scary. Will these pathogens lead to minor nuisance illnesses like the common cold or horrible consequences like EBOLA, which kills nearly 90% of its victims? … or be more like the flu? Most people can understand and calibrate the severity of a virus based on their experience with the flu. Over the last decade, based on Center for Disease Control (CDC) statistics, the flu infects between 3-15% of all Americans each (mostly winter) season. Between 1 and 2% of flu victims require hospitalization and between 0.1 and 0.2% of victims die of complications of the illness. Death from the flu occurs mostly in infants, the very old and in folks with immune deficiency or other significant chronic illness.
For COVID-19, the initial reports of death rates of 4% in China and 10% in Iran now appear to reflect poor reporting (local officials and our media) and selective testing of patients. To make an accurate estimate of a death rate you need to know the total number of tests administered (which the media doesn’t typically report) to get a sense of whether all of the positive + tests are being captured. Otherwise, the death rate reflects what happens only to the sickest patients, those already on death’s door when they receive testing, rather than the full spectrum of disease.
As of this past weekend, we have good data to review to help us understand the true death rate of COVID-19. As of March 2, in South Korea, a hard hit country with a good healthcare system, the death rate is 0.51% (< 1%). South Korea has deployed extensive resources for testing of coronavirus. South Korea has now reported 22 deaths occurring among 4,335 patients infected by COVID-19 out more than 100,000 patients tested. The large number of tests and the relatively low number of positive tests helps us feel confident that South Korea has identified most patients with the illness, an essential part of the equation needed to determine the true death rate.
The South Korean death rate likely reflects a maximum rate of death. We suspect that the death rate will be lower in the US since we have had more warning and will intervene earlier with antiviral medications and support.
The low death rate is good news. Unfortunately, this good news has a down side. Because of the mild illness that results from infection in most folks who contract it, COVID-19 will likely spread extensively before it winds down. Ironically, there is a tradeoff between viral spread and lethality. The worst viruses, like EBOLA, kill most infected people, but do not spread widely because people get sick quickly and have far fewer contacts. With the flu or COVID-19, people may have minor symptoms that allow them to function in society and spread the virus. Governor DeSantis of Florida announced the first confirmed cases in the state on Sunday (March 1) and more cases will certainly occur, probably many more cases.
Another part of our mission at ENCORE involves helping to get new medical therapies to patients. We participate as an active research site in the medical product development system. We have already received contact about our ability to test a coronavirus vaccine in healthy patients wishing to avoid illness. These studies focus on prevention. We have assured the manufactures of the vaccines that we are poised and ready to jump in for the clinical study.
Many antiviral drugs “sit on the shelves” of pharmaceutical companies that have had proven efficacy against the SARS and MERS viruses – similar in structure but more deadly than COVID-19. We have no reason to believe that some of these drugs will not work against COVID-19, but they remain untested. At this time, since we do not have any patients with COVID-19 at our clinics, we will not participate in the treatment studies. However, if things change, we will respond.
So, what to do now?
If you would like to be on standby as a volunteer for a healthy patient vaccine study let us know. Contact – 904-730-0166 or Jaxresearch.com. In the meantime, wash your hands like crazy, keep hand sanitizer in the car and/or office, and use it at least 5 times a day during cold and flu season!
Michael J. Koren, MD, FACC, CPI