Dependent vs Independent Variables, How to Tell the Difference
Listen to the article here:
In science and medicine we measure if and how well things work using measurements. This idea may sound simple, but it’s often a challenge to find out exactly what to measure – and how. We typically measure things that can change – things that can vary. We call these things variables. Variables can be broadly split into two major categories: dependent and independent. Either type of variable can change, the difference is what changes them.
Independent variables are changed by researchers, particularly in clinical (patient) research. This variable in a medical research study is what we are testing. The changes to an independent variable may include dose, length, and method of drug delivery. We evaluate independent variables that may change outcomes of the people in a study – but sometimes they do not. In order to understand the effect of medicines, researchers test the medicine against a control. The control could be a placebo (something that has no effect) or a standard of care (the current normal medicine).
Dependent variables are what we expect to change during a trial. In a clinical research, we may expect changes in blood pressure, cholesterol levels, disease symptoms, mortality, and other categories. In a well designed study, we assess changes in the dependent variables related to changes in the independent variables. There is always the chance that the dependent variables are changed by other things, however. A patient might take a new blood pressure medicine but retire from their job. The reduced stress could decrease their blood pressure even if the medicine did not.
Because of individual changes in people’s circumstances, researchers use statistics to find trends. If your blood pressure medicine was only studied on the one person above, you might have erroneous results. Instead clinical trials have dozens, hundreds, or even thousands of participants. With large populations these little differences get figured out. One person might retire, but another might get fired, having an opposite effect. Altogether, statistical analysis can help discover if any changes in the dependent variable are due to the effects of the independent ones.
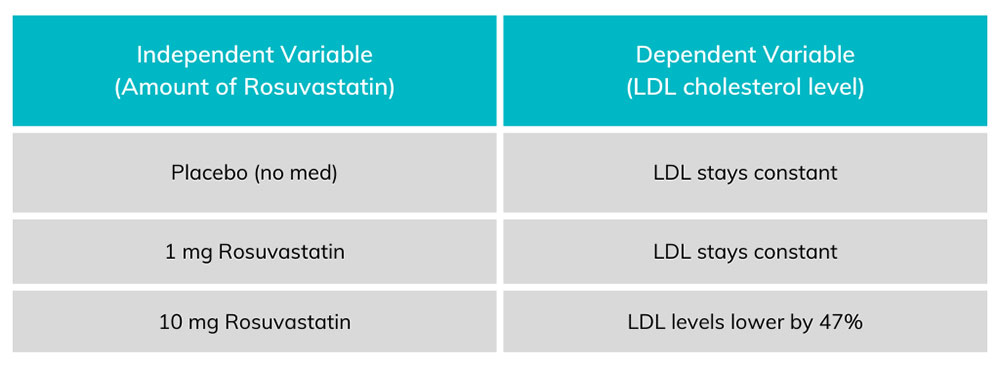
Chart 1. Each amount of Rosuvastatin on the left corresponds to an amount of LDL on the right. The dependent variable (LDL levels) change in proportion to the amount of independent variable (rosuvastatin) taken by the patient.
Other variables exist in a study. The most concerning of these variables is known as a confounding variable. This is a variable that can undermine the study at a fundamental level. A confounding variable can be introduced by researchers and might include things like placing all overweight patients in the 10 mg group and all underweight people in the placebo group. ENCORE Research Group (and any legitimate clinical research group) avoids confounding variables and bias by randomizing patients. Patients are randomized through an impartial method (usually a computer program) which will randomly place patients into any of the test groups. By randomizing patients, we can avoid the most concerning confounding variables and make sure we are studying what we intend to!
To learn more about the clinical trial process, call our Recruitment Team at (904) 730-0166.
Written by Benton Lowey-Ball, BS Behavioral Neuroscience
Sources:
Schweiger, C. (2003). Clinical trials with rosuvastatin: efficacy and safety of its use. Italian Heart Journal: Official Journal of the Italian Federation of Cardiology, 4, 33S-46S. https://pubmed.ncbi.nlm.nih.gov/14983745/
Stewart, P. A. (1978). Independent and dependent variables of acid-base control. Respiration physiology, 33(1), 9-26. https://www.nlm.nih.gov/nichsr/stats_tutorial/section2/mod4_variables.html

