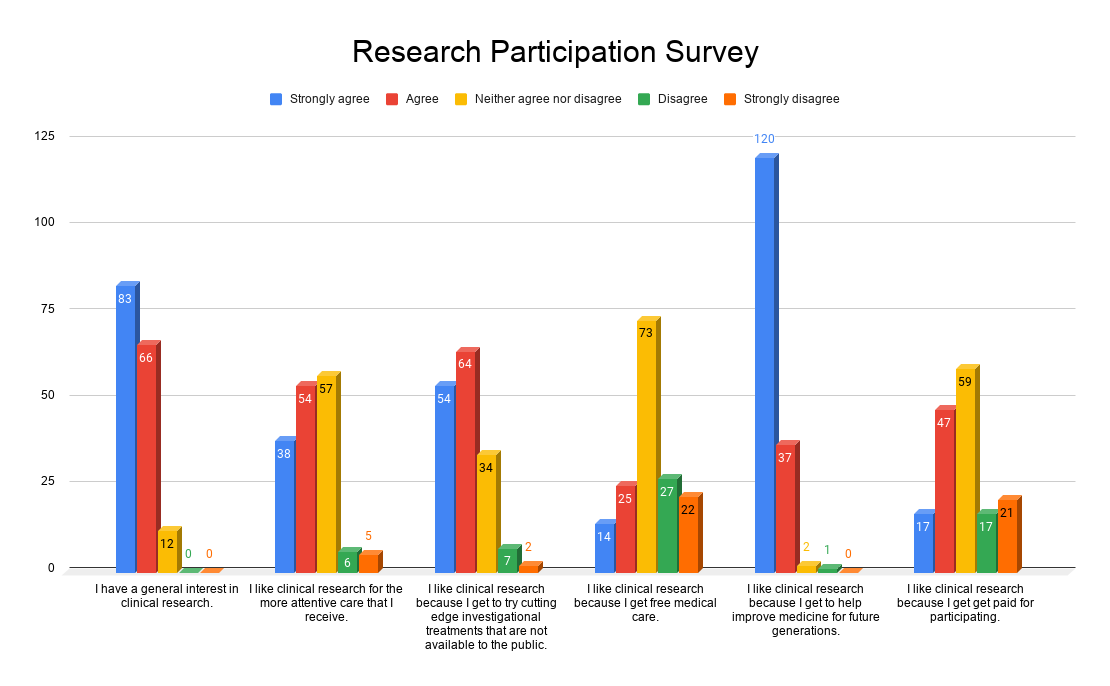
There are many reasons someone may think it’s scary to participate in a clinical trial. However, if you become more informed about how clinical trials work then those fears will vanish! Below are some common misconceptions about clinical trial participation.
“I won’t be able to quit if I change my mind about participating.”
Many believe that once you sign the informed consent papers to participate, you are locked in until the trial is complete. This is a common misconception. Clinical trials rely on participants who volunteer. You are always able to leave a clinical trial. After you have taken an investigational injection or drug, you are allowed to leave. However, it is important to be open and honest with your clinical team about leaving the trial.
Research participants are guinea pigs.
Thinking about yourself strapped down in a lab while people poke you with needles sounds like a scene right out of a horror movie. It is far from the truth. There are strict guidelines for each clinical trial, and all require special attention to health concerns. There are also ethical and moral guidelines put in place by a committee for each trial, called the Institutional Review Board (IRB). This is a committee made up of health professionals to ensure each trial is ethical. There is a robust screening and preclinical testing process which can take up to six years to complete before a drug is ever given to a patient.
Will it Hurt?
This depends on the type of clinical trial you are participating in. Each process is different. The trials can include taking an investigational pill, undergoing a new procedures or getting an injection. One thing you can be sure of, your health will be closely monitored and you will be aware of all risks and procedures before participating in the trial. You will review and sign the “informed consent” document. This will ensure you know all the risks. The document ensures the patient knows everything involved and is free to choose whether or not to participate. If you ever feel unsure, do not be hesitant to ask your provider any questions!
Will I experience major side effects?
There is some risk when participating in a clinical trial.An investigational drug goes through a robust testing process. Before is is given to a patient, the results must suggest that it is highly likely to be safe and effective. Patients are closely monitored once given an investigational drug to ensure their safety. The informed consent and IRB both take part in ensuring the safety and ethics of every clinical trial.