GRID VIEW
What happens when the things that are supposed to keep us safe turn against us? In fiction, this is usually posed as some big, world-ending problem like artificial intelligence going off the rails and killing everybody. In nonfiction, this question is hashed out in the world of autoimmune diseases. Systemic Lupus Erythematosus (SLE) is an autoimmune disease that typifies an entire cluster of autoimmune diseases , known as “lupus-associated”. These include rheumatoid arthritis, systemic sclerosis (scleroderma), Sjogren’s syndrome, and more. These diseases tend to spread though different parts of the body, which is why many have “systemic” in the name. Systemic lupus erythematosus is a chronic and progressive autoimmune disease. Chronic indicates that it is long-lasting, and progressive means that the disease can change over time, usually by getting stronger and more severe. It is much more prevalent in racial and ethnic minorities, and 90% of sufferers are women. These statistics immediately give us clues as to the nature of SLE and autoimmune diseases: they have a genetic component. People from different parts of the world have different packages of genes, but why are women so susceptible? Genes are part of our DNA, the code that describes what we are. Specifically, they are sections of code that describe how to build a protein. Each gene has instructions for a single protein, and changes to the genetic code can result in changes to the protein that it’s supposed to build. Genes aren’t randomly distributed in the DNA code; they are located on chromosomes. We have 23 chromosomes that we get from our mother and 23 from our father. Most are the same size, but the X and Y sex chromosomes look different. The X chromosome, attributed to females, contains 800-900 genes while the Y chromosome, attributed to males, contains only 50-60 genes! It is thought that a genetic component of autoimmune diseases may be found on the X chromosome. The immune system is made of cells that have specific proteins they use to identify invaders, send alert signals, and attack. A huge class of signaling molecules is called hormones. Many of these are proteins. One class of immune hormone proteins is interleukins. When there are too many of these, they are in the wrong place, or they have changed in some way the immune system can go haywire and attack healthy cells. Autoimmune diseases like SLE are complex. The immune system gets out of whack due to multiple conditions acting together. Affected people have a genetic predisposition: their DNA has code that makes it likely to produce dangerous levels or types of interleukins. This isn’t a foregone conclusion, however. An environmental stimulus is needed to start the autoimmune process. This can be airborne particles like silica or cigarette smoke, drugs including contraceptives, viruses, and even sunlight! When the genetic primer is lit, the immune system misidentifies what is good and bad and can explode on healthy cells. Like many autoimmune diseases, SLE has a cycle of remission and relapse; people have symptom-free periods, and periods of increased symptom activity. Symptoms may be low on the disease scale, including pain, fatigue, and a rash on the face. This rash may be exacerbated by UV light, appearing on the nose and cheeks where we get sun. This takes on the classic “butterfly rash” shape associated with SLE. Higher on the disease scale is damage to organs like the kidneys, heart, lungs, bloodstream, gut, and nervous system. When it is widespread it can also cause skin and joint disease, including arthritis. Severe cases can lead to hospitalization and death. So what can we do about autoimmune diseases like SLE? Each patient is different, each disease is different, and doctors have to balance the effects of medications against side effects. In general, the most obvious preventative step for autoimmune diseases is something that calms the immune system down. Antimalarials like hydroxychloroquine decrease immune activity and are often a preventative step. During flare-ups, a targeted anti-inflammatory like a glucocorticoid may be used. If these fail, doctors may prescribe immunosuppressant drugs or monoclonal antibodies. Specific organ maintenance, like treating liver and kidney problems can help alleviate damage, and doctors can also recommend procedures that work directly on our bloodstream. The future may hold promise for new medications that target specific parts of the autoimmune disease pathway. Keep your eyes open for new clinical trials aimed at helping those with autoimmune diseases like SLE. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: Ameer, M. A., Chaudhry, H., Mushtaq, J., Khan, O. S., Babar, M., Hashim, T., … & Khan, O. S. (2022). An overview of systemic lupus erythematosus (SLE) pathogenesis, classification, and management. Cureus, 14(10). https://doi.org/10.7759/cureus.30330 Angum, F., Khan, T., Kaler, J., Siddiqui, L., & Hussain, A. (2020). The prevalence of autoimmune disorders in women: a narrative review. Cureus, 12(5). https://doi.org/10.7759/cureus.8094 Barber, M. R., Drenkard, C., Falasinnu, T., Hoi, A., Mak, A., Kow, N. Y., … & Ramsey-Goldman, R. (2021). Global epidemiology of systemic lupus erythematosus. Nature Reviews Rheumatology, 17(9), 515-532. https://doi.org/10.1038/s41584-021-00668-1 Mackay, I. R. (2009). Clustering and commonalities among autoimmune diseases. Journal of autoimmunity, 33(3-4), 170-177. https://doi.org/10.1016/j.jaut.2009.09.006
Scroll down to listen to this article.
Listen to the article here:
I remember learning in biology that we’re made out of cells. That’s true, but not all the way true. We’re also made out of the stuff in between cells This stuff is called the extracellular matrix. Extracellular means outside of the cells and matrix in this context means the environment that is occupied. Though the extracellular matrix isn’t a dystopian cyberworld made by robots, it can be dangerous if parts of it start getting out of control. Systemic sclerosis, a type of scleroderma, is a disease that results in excess material being deposited in the extracellular matrix, causing body-wide problems. Systemic sclerosis is a rare, chronic, and progressive autoimmune disease. It affects women in their 60s, though men and African Americans have the worst outcomes from the disease. Around one in four people with systemic sclerosis also have another autoimmune disease. The biggest risk factor for systemic sclerosis is family history; and genetics. It is thought that some people have a genetic predisposition to the disease and that environmental factors cause it to “kick on.” As an autoimmune disease, systemic sclerosis is complex. It may present differently in each patient who has it. One of the easiest ways to differentiate types is by how it affects skin. Sclerosis comes from Greek and means to harden or a tumor. Systemic sclerosis is hard, tumor-like skin that affects the whole body (or system). There are three categories based on how widespread the condition is. Systemic Sclerosis sine scleroderma is when skin is unaffected, though fingers may experience discoloration. Limited cutaneous systemic sclerosis is, as the name suggests, limited. The skin on the fingers and face is affected. It tends to progress slower and may be less dangerous. Diffuse cutaneous systemic sclerosis is quite the opposite. It is diffuse, meaning it spreads far and wide. It may affect the arms and legs up to knees and elbows, the chest, stomach area, and back. It progresses quickly and is associated with poorer outcomes. Beyond the hardening of the skin, there are other symptoms, and (unsurprisingly) none are great. Patients may experience: All of these can be annoying and painful, but many are very dangerous. Systemic sclerosis, in fact, is the most deadly of all rheumatic diseases. Let’s dig into how systemic sclerosis works to find out why. As stated before, systemic sclerosis is an autoimmune disease. Some environmental factors like smoke, alcohol, viruses, solvents, or chemicals activate our epithelial cells. Epithelial cells interact with the outside world and are located on the skin, in our lungs and digestive tract, and around organs. The cells send out danger signals in the form of cytokines. There are several cytokines, but one of the most prevalent in this system is type 1 interferon (IFN). If this were The Matrix these guys would be the Agents, good at getting rid of unwanted intruders but dangerous when they get out of control. They are usually sent out in viral infections and put the immune system into a general state of alert. When there are too many cytokines, they start causing damage that needs to be repaired. The body responds to high IFN in a couple of ways: alerting immune cells, starting inflammation, and – in people with systemic sclerosis, by activating fibroblasts. Fibroblasts make the connective tissue between cells (you may see where this is going). These guys go into overdrive and spew extra collagen, elastin, and other connective tissues between cells. They try to repair damage from the cytokines, but the excess tissue signals problems and creates a deadly feedback loop. When this is bad enough, normal cells are replaced by this dense tissue in the extracellular matrix. The skin on the fingers and toes thickens, and the vascular (circulatory) system starts getting crushed. Small blood vessels called capillaries die. The tissue around your vital organs thickens and causes damage. The body can’t deliver enough oxygen to organs, which is bad. If this happens to the blood vessels of the lungs, it’s even worse. The thickening on the fingers, toes, arms, legs, and face may be debilitating, but the real danger is what’s happening inside. So what can be done? Doctors can try to treat the symptoms and complications to keep people comfortable. They may treat vascular, skin, kidney, arthritic, and gastrointestinal problems. If the lungs are affected, specific monoclonal antibodies may be prescribed. Some patients also find that general immunosuppressors help, but these can come with myriad side effects. The best bet for patients is to discover and treat systemic sclerosis early, before damage spreads, if possible. Medications may slow the decline, possibly until the disease becomes stable. On the horizon, clinical researchers are looking at medications that may cut the feedback loop and help patients break free from the matrix. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: Harrison, D. G., Coffman, T. M., & Wilcox, C. S. (2021). Pathophysiology of hypertension: the mosaic theory and beyond. Circulation research, 128(7), 847-863. https://doi.org/10.1161/CIRCRESAHA.121.318082 Myat, A., Redwood, S. R., Qureshi, A. C., Spertus, J. A., & Williams, B. (2012). Resistant hypertension. Bmj, 345. https://doi.org/10.1136/bmj.e7473 Sarafidis, P. A., Georgianos, P., & Bakris, G. L. (2013). Resistant hypertension—its identification and epidemiology. Nature Reviews Nephrology, 9(1), 51-58. https://www.nature.com/articles/nrneph.2012.260
Scroll down to listen to this article.
Listen to the article here:
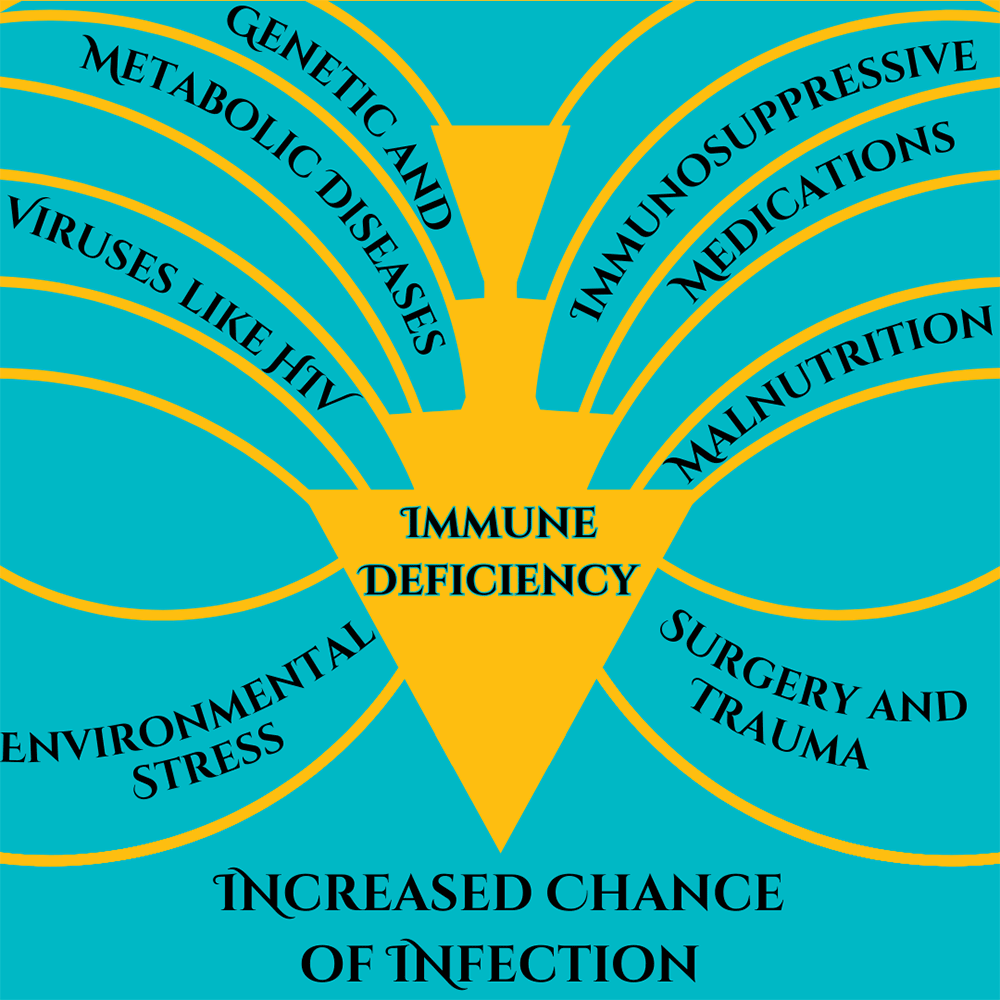
I remember the early days of COVID-19, everything was new and scary and dangerous and no one knew what was going on. It seemed to be very dangerous for two groups of people, those of advanced age and those who were immunocompromised. Vaccines rolled out, testing became easy, and things have calmed down quite a bit. But corona isn’t all limes on the beach, as any virus, it’s still dangerous for people who are immunocompromised. What does this term mean, who are the immunocompromised, and is there anything they can do in the new reality of COVID-19? Immunocompromised is a broad term. It indicates that a person’s immune system cannot generate an appropriate response to infection. When bacteria or viruses make it inside these people’s bodies, they cause much more damage and are very hard to control. The immune system is very, very complicated, so there are many ways a person can become immunocompromised. We can generally lump these into two categories: primary and secondary immunodeficiency. Primary immunodeficiency means the condition is built-in to the body; it’s genetic. Primary immunodeficiencies are fairly rare, with <0.1% of the population experiencing them. The rest of the almost 3% of people who have immunodeficiencies have secondary immunodeficiencies.Infectious diseases (such as HIV), malnutrition, age, surgery, environmental stress, and immunosuppressive drugs can all cause secondary immunodeficiency. Immunodeficiency affects millions of Americans. Women are twice as likely as men to have immunodeficiency; it is most common in white Americans and those aged 50-59. Nearly 3% of the population – over 9 million Americans – are suspected to have immunodeficiency. Unfortunately, immunodeficiency can greatly reduce a person’s ability to deal with a COVID-19 infection. The most obvious problem is that immunocompromised people are more susceptible to severe symptoms. A disproportionate amount of people who are hospitalized for COVID-19 are immunocompromised. Immunodeficiency doesn’t compromise, it packs a double-whammy. Those with a weak immune system also find vaccines less effective! In fact, 44% of people who had “breakthrough” cases (where they were vaccinated but still hospitalized) were immunocompromised. This is because the body is unable to produce enough protective antibodies for the body to be protected – a process called seroconversion. Seroconversion is when antibodies are able to be detected in the blood. With vaccines, successful seroconversion indicates that the body is protected and has the equipment necessary to put up a good fight against the COVID-19 virus. When vaccinated against COVID-19, people with healthy immune systems showed seroconversion rates of 99%. The type of immunocompromisation affects how well vaccines produce seroconversion. People with solid tumor cancers, such as breast, colon, prostate, and lung cancer show seroconversion rates of 92%. Immune-inflammatory disorders like lupus, primary biliary cholangitis, psoriasis, and rheumatoid arthritis have seroconversion rates reduced to 78%. Vaccine effectiveness in people with blood cancers such as lymphomas, myeloma, and leukemia drops to 64%. Those with organ transplants have to be on strong immunosuppressive drugs to avoid organ rejection and because of this they have the lowest rates of seroconversion, only 27%.
Some factors of immunocompromisation. Adapted from Chinen, J., & Shearer, W. T. (2010).
So what can immunocompromised people do to protect themselves against COVID-19? A lot of the same things as people who are immunocompetent! High quality masks and respirators can help. Avoiding crowds and indoor areas with poor ventilation is a must. Washing hands with soap and water is critical, though hand sanitizer is a good second option. Immunocompromised people who contract COVID-19 should contact their doctor or other healthcare provider right away. Isolating and using masks to prevent the spread is always a good idea. Immunocompromised people may also keep infections from getting out of control if their medical provider recommends an antiviral medication or convalescent plasma. Of course, the best way to avoid getting sick with COVID-19 is through prevention, including vaccines. A 27% seroconversion rate is much better than 0% after all. And there may be more hope for immunocompromised people, as new vaccines are being developed to serve this community. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: Boyle, J. M., & Buckley, R. H. (2007). Population prevalence of diagnosed primary immunodeficiency diseases in the United States. Journal of clinical immunology, 27, 497-502. https://link.springer.com/article/10.1007/s10875-007-9103-1 Chinen, J., & Shearer, W. T. (2010). Secondary immunodeficiencies, including HIV infection. Journal of Allergy and Clinical Immunology, 125(2), S195-S203. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6151868/ Harpaz, R., Dahl, R. M., & Dooling, K. L. (2016). Prevalence of immunosuppression among US adults, 2013. Jama, 316(23), 2547-2548. https://jamanetwork.com/journals/jama/fullarticle/2572798 National Institute of Health. (July 21, 2023). Special considerations in people who are immunocompromised. COVID-19 Treatment Guides, https://www.covid19treatmentguidelines.nih.gov/special-populations/immunocompromised/ Parker, E. P., Desai, S., Marti, M., Nohynek, H., Kaslow, D. C., Kochhar, S., … & Wilder-Smith, A. (2022). Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. The Lancet Global Health, 10(3), e326-e328. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(21)00593-3/fulltext SY, L. A. W., SC, C. L. L., & Muthiah, L. M. (2021). Efficacy of COVID-19 vaccines in immunocompromised patients: A systematic review and meta-analysis. medRxiv. https://doi.org/10.1136/bmj-2021-068632
Scroll down to listen to this article.
Listen to the article here:
When patients are diagnosed with an autoimmune disorder they often have many questions. How did this happen? What is happening inside me? What treatments are available? Autoimmune diseases can be extremely complex and are the subject of much current research. The immune system’s purpose is to identify and destroy threats to the body such as viruses, bacteria or parasites. However, when a person has an autoimmune disease such as Crohn’s Disease, Lupus, Sjogren’s (show-grins) syndrome the immune system becomes unable to distinguish foreign bodies from the body’s own healthy tissue. When this happens, the immune system begins to target healthy cells causing inflammation. Almost any aspect of the immune system can malfunction causing a plethora of conditions.
One such condition is Crohn’s Disease. Crohn’s is an autoimmune disease where the immune system specifically targets the gastrointestinal tract. Crohn’s can be difficult to diagnose due to the variety of symptoms associated with the disease. The symptoms vary from person to person and by which component of the GI tract is being targeted. If a doctor suspects Crohn’s Disease, diagnosis is confirmed via an upper and/or lower endoscopy. Those living with Crohn’s disease will agree that we need to find a cure ASAP!
Systemic Lupus Erythematosus(SLE) is a chronic autoimmune disease in which the immune system can cause damaging inflammation to any part of the body. Skin, joints and organs can all be affected. Flares can cause a wide variety of symptoms. Around half of those affected by lupus have what is called a butterfly rash on their face. Other common symptoms include inflammation or swelling of the joints, and fatigue.
Another inflammatory autoimmune disease is Sjogren’s Syndrome. Sjogren’s is typically identified by its most prevalent symptoms, which are dry eyes and dry mouth. These symptoms occur because the immune system targets the glands that produce saliva.1 In the past treatment has almost entirely consisted of treating the symptoms of the disease. However, new research is showing that Sjogren’s can lead to other complications and scientists are now working on specially devised treatments to nip the problem in the bud!
According to JCCR’s Steven Mathews, MD “the last generation of autoimmune treatments worked further down the mountain so to speak and focused on treating the avalanche of symptoms. Current treatments are looking at treating conditions higher up the mountain at the source and preventing the avalanche from occurring.” Richard Smith, RN elaborated that, “general immunosuppressants act like a hammer on the immune system, whereas the current drugs we are researching act like a fine scalpel only targeting the rogue immune cells.” Our mission at ENCORE Research Group has always been to help get cutting edge treatments approved by the FDA. We want to help deliver safe and effective treatments to everyone. This is only possible by conducting research studies on new investigational medications. If you are interested in taking part in one of our research studies call today.