GRID VIEW
As an avid surfer, I occasionally hear concerns about shark attacks at the beach. Diving into the statistics of unprovoked shark attacks, I learned that they are astronomically unlikely. Deaths are even rarer, with only around one fatal shark attack per year in the U.S. So then what is the deadliest animal? Worldwide, scorpions kill a few thousand people annually, dogs around ten thousand annually, and snakes kill some 75,000 people a year! That’s a drop in the bucket compared to other humans, who kill around half a million people per year. But then there are mosquitoes. Mosquitoes kill more people than every other animal combined – including humans; something like 750,000 to 1 million people per year. Let’s get the buzz on why.
Mosquitoes don’t kill us directly. At least not normally. Sometimes blood loss from mosquito bites can kill animals as big as cows, but this is an exception rather than the rule. Normally, mosquitoes kill people by acting as vectors, which transmit disease. The deadliest known disease in the history of the planet is malaria, and it is responsible for at least several billion deaths throughout history (the exact number is quite controversial, with estimates ranging from 5% – 50% of all people ever to live). Mosquitos also transmit dengue, yellow fever, chikungunya, zika, and more. The question then is… why? Why us in particular?
Actually, only some mosquitoes like humans in particular. There are around 3,600 types of mosquito. Some species, like Aedes aegypti (a-ee-dees a-gyp-thai), hunt humans specifically for blood. Others target snakes, frogs, or birds. Many are generalists and hunt anything with blood. However, consuming blood is actually a rare occurrence in the life of a mosquito. For most of their lives mosquitoes are vegetarians. They eat plant nectar, fruits, and the sugary waste of aphids called honeydew. Mosquitoes pollinate flowers, like to eat apples and bananas, and wanna hang out for a nice long walk on the beach. Some mosquito species actually stay vegetarian their whole lives. In fact, male mosquitoes don’t consume our blood, it’s only females when they need to lay eggs. Fruit juice is nice, but – as every good vegan knows – you need to get your protein somehow. For mosquitoes, some species need the extra protein found in blood to help their young thrive. How mosquitoes actually locate a host is pretty complex.
It’s easy to guess how a mosquito might find us by looking at what signals we give off. We breathe, we smell, we’re warm, we look like people, and we taste like humans. Each of these features attract mosquitoes from progressively shorter distances. Let’s move through how.
- Breath
- When we exhale, CO2 comes out. These puffs of carbon dioxide travel through the air, dispersing into relatively big clouds. Mosquitoes have a special nerve cell called a cpA neuron that can detect CO2. Mosquitoes follow the trail of CO2 upwind until they smell us.
- Odor
- Mosquitoes can detect the specific scent profile animals emit using those same cpA neurons. They then determine if the smell matches the creatures they prefer to hunt using their antennae and other nose-like organs. Humans emit a lot of scents. Key among these are acetoin, made by skin bacteria, and volatile carboxylic acids, like lactic acid. The amount and composition of these chemicals change based on genetics and environmental changes. Having malaria, for instance, makes you smell more attractive to mosquitoes.
- Temperature
- When a mosquito gets close enough, it can start detecting body heat, which draws them in.
- Shape and Color
- Mosquitoes use vision to detect us from a few inches away. Their eyes are specialized to detect redder wavelengths of light, similar to many skin tones, and they preferentially fly towards high contrast objects: think a dark arm against a bright blue sky.
- Taste
- The last step before ruining our outdoor fun is to make sure we taste good. Rubbing disgusting-tasting bug spray all over our bodies helps keep mosquitoes from wanting to eat us, but normally they’re way into the taste of old skin and sweat.
People exhibit variations on all these areas (except for breathing). Our smells change, some of us wear insulating clothes, skin tones vary, and according to Dr. Hannibal Lecter, we taste different. Scientists have studied the variation between people and how many mosquitoes bite them in an effort to seek relief from mosquito bites. Mosquitoes tend to bite pregnant individuals more frequently and genetics play a role, but these factors are difficult to alter in many people. Instead, researchers tend to target our most modifiable attractant, smell. Our skin microbiome and genes affect our scent, but diet seems to as well – though not as much as many people claim. Randomized clinical trials have found no evidence that vitamin B, garlic, and green grapes affect mosquito bites. There is some preliminary evidence pointing to caffeine as a possible attractant. Studies have found evidence that eating bananas and drinking beer both increase mosquito interest. As stated before, having malaria makes you more attractive to mosquitoes. Unfortunately, you may have noticed that none of these reduce our attractiveness to mosquitoes.
Bug spray containing DEET makes it more difficult for bugs to smell you and is recommended, but can be sticky, stinky, and unpleasant to use. Next-generation bug repellents may block multiple scents or even inhibit the cpA neurons directly! Physical barriers like long sleeves can help as long as they don’t overheat you. Really, the problem is best summarized in a paper by Van Breygel et al. (2015):
For a human hoping to avoid being bitten by a mosquito, our results underscore a number of unfortunate realities. Even if it were possible to hold one’s breath indefinitely, another human breathing nearby, or several meters upwind, would create a CO2 plume that could lead mosquitoes close enough to you that they may lock on to your visual signature. The strongest defense is therefore to become invisible, or at least visually camouflaged. Even in this case, however, mosquitoes could still locate you by tracking the heat signature of your body provided they get close enough. The independent and iterative nature of the sensory-motor reflexes renders mosquitoes’ host seeking strategy annoyingly robust.
The obvious reaction to this is to think “kill ‘em all!” Unfortunately, even this method fails. Insecticides have a nasty habit of prompting natural selection to favor bugs immune to them – and they manage to kill many innocent bugs in the process. Traps have limited effectiveness, can be expensive, and also manage to murder countless other ecologically important bugs. With this in mind, perhaps the solution to saving lives from the world’s deadliest animal isn’t in reducing our attractiveness (my mom tells me I’m very attractive), but in reducing our susceptibility to the diseases they carry. Across the globe, scientists are in various stages of research seeking vaccines for malaria, dengue, and other mosquito-borne diseases. If these manage to be successfully tested and distributed, maybe we won’t have anything to fear from mosquitoes after all! Except for the itching. And the annoyance. And the constant ankle biting. And that they like to fly at our eyeballs. And that they might literally take more blood out of us than those sharks everyone tells me to watch out for.
Staff Writer / Editor Benton Lowey-Ball, BS, BFA
Listen to the article here:
References:
Associated Press. (September 9, 2020). Thick clouds of mosquitoes kill livestock after hurricane. https://apnews.com/article/horses-animals-insects-storms-hurricane-laura-fa0d05b046357864ad2f4bb952ff2e3e
CDC Global Health Center. (April 8, 2024). Fighting the world’s deadliest animal. Centers for Disease and Control. https://www.cdc.gov/global-health/impact/fighting-the-worlds-deadliest-animal.html
Brown, J. E., Evans, B. R., Zheng, W., Obas, V., Barrera-Martinez, L., Egizi, A., … & Powell, J. R. (2014). Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution, 68(2), 514-525. https://academic.oup.com/evolut/article/68/2/514/6852391
Ellwanger, J. H., da Cruz Cardoso, J., & Chies, J. A. B. (2021). Variability in human attractiveness to mosquitoes. Current Research in Parasitology & Vector-borne Diseases, 1, 100058. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8906108/
U.S. Environmental Protection Agency. (September 25, 2023). Insect repellents: DEET. https://www.epa.gov/insect-repellents/deet
Giraldo, D., Rankin-Turner, S., Corver, A., Tauxe, G. M., Gao, A. L., Jackson, D. M., … & McMeniman, C. J. (2023). Human scent guides mosquito thermotaxis and host selection under naturalistic conditions. Current Biology, 33(12), 2367-2382. https://www.cell.com/current-biology/abstract/S0960-9822(23)00532-8
Peach, D. A., & Gries, G. (2020). Mosquito phytophagy–sources exploited, ecological function, and evolutionary transition to haematophagy. Entomologia Experimentalis et Applicata, 168(2), 120-136. https://doi.org/10.1111/eea.12852
Potter, C. J. (2014). Stop the biting: targeting a mosquito’s sense of smell. Cell, 156(5), 878-881.https://www.sciencedirect.com/science/article/pii/S0092867414001585
Raji, J. I., & DeGennaro, M. (2017). Genetic analysis of mosquito detection of humans. Current opinion in insect science, 20, 34-38.https://www.sciencedirect.com/science/article/pii/S2214574517300342
Shen, H. H. (2017). How do mosquitoes smell us? The answers could help eradicate disease. Proceedings of the National Academy of Sciences, 114(9), 2096-2098 .https://www.pnas.org/doi/10.1073/pnas.1701738114
Tauxe, G. M., MacWilliam, D., Boyle, S. M., Guda, T., & Ray, A. (2013). Targeting a dual detector of skin and CO2 to modify mosquito host seeking. Cell, 155(6), 1365-1379. https://www.cell.com/cell/fulltext/S0092-8674(13)01426-8
Van Breugel, F., Riffell, J., Fairhall, A., & Dickinson, M. H. (2015). Mosquitoes use vision to associate odor plumes with thermal targets. Current Biology, 25(16), 2123-2129.https://www.sciencedirect.com/science/article/pii/S096098221500740X
Scroll down to listen to this article.
Though it’s still warm and beautiful out, winter looms on the horizon. Winter in Florida can be good: a time for family, biking and outdoor exercise, bigger waves for surfing, and tasty foods. Winter can also be bad: a time for cold, wetsuits, fruitcakes, and the flu. So what is the flu, how does it work, and why does it take after my extended family and only visit us in the cooler months?
The seasonal flu is caused by the flu virus, properly called Influenza, and more properly a type of orthomyxoviridae (there will be a test at the end). There are three categories of influenza viruses that infect humans, conveniently named influenza A, B, and C. These are distinct from each other in some critical ways, one being how easily they change the proteins on the outside of the virus. Viruses are tiny little packets of DNA or RNA that are contained in a little pouch. The pouch has special proteins called antigens on the outside that help it invade target cells. The proteins are also one of the key ways our immune system detects and fights these viruses. Influenza A changes these rapidly, influenza B changes slowly, and influenza C is stable, undergoing little or no change over time.
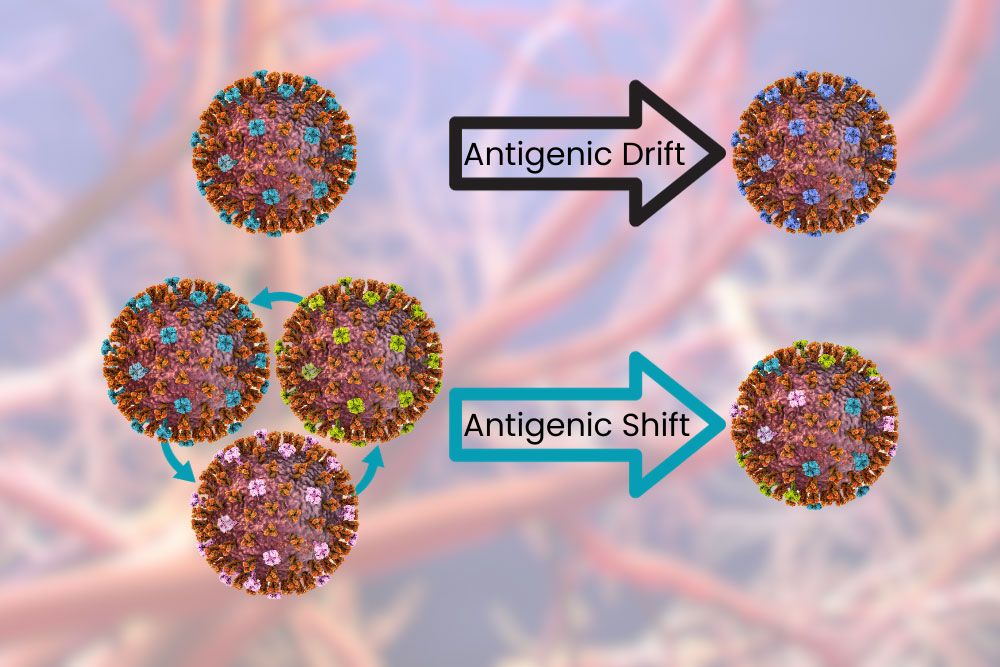
Influenza A and B viruses undergo a process called antigenic drift. This is when the surface proteins change a little bit at a time. The changes can cause incremental “improvements” to the virus, allowing it to evade our immune system and infect cells more easily. These changes are fast enough that over the course of a year your body may not be able to recognize the virus and you may get the flu year after year, but slow enough that you probably won’t get it twice in the same season – especially with a vaccine. Influenza A can also undergo antigenic shift, which is like the antigenic drift on overdrive. This is like when your dad shaves his beard for the first time in 20 years: same thing underneath but different enough you have trouble recognizing him. When this happens your body can’t recognize the virus as dangerous and previous antibodies and vaccines provide little or no aid. Because of this Influenza A has been responsible for all flu pandemics.
Why is it seasonal though? Well, it’s not seasonal everywhere. In some tropical and subtropical areas, the flu follows the rainy season and comes twice a year. In some tropical areas the flu is present year-round. This provides a clue; it’s the weather! Strangely, it might actually be our response to changing weather that is the progenitor of a flu season. Influenza viruses spread through the air. This is bad news for us in that they spread easily from person to person, but also means they are affected by the weather more than things that may be spread by fluids or insects. Temperature and relative humidity are the two biggest factors. Cool, dry air gives influenza viruses a better chance for infecting us. The virus is more stable in droplets, more of them are shed, and the droplets might stay in the air longer as they evaporate. Our defensive capabilities are decreased in the cold – anyone with a dry throat can attest to this. The protective mucus layer in our airway is decreased and the innate immune system isn’t as efficient. To make things worse, in the winter we spend more time indoors.
In the industrialized world, including America, we spend almost no time outdoors. A 2001 study found that we spend around 87% of our time in buildings and 6% in cars. This varies heavily by where you work, but most of us spend almost our whole lives in buildings with recirculated, conditioned air. In the winter, heated air is drier, which promotes influenza virus stability. As we walk into and out of buildings our throats are dry and inefficient at removing viral particles. This creates ideal conditions for Influenza to infect and spread during the winter.
So what can we do? Get vaccinated of course! Every year scientists work hard to produce vaccines that will target the most likely versions of influenza to emerge during the winter. Antigenic drift is taken into account and vaccines will (hopefully) protect against the likely minor antigenic changes between production of the vaccine and emergence of flu season. New mRNA vaccines shorten the time between production and deployment of vaccines based on the current dominant strain, increasing their effectiveness. All flu vaccines available in the USA are quadrivalent, meaning they protect against two strains of influenza A and two strains of influenza B. So don’t forget to get a flu vaccine, spend time with the family, and go outside!
Staff Writer / Editor Benton Lowey-Ball, BS, BFA
Listen to the article here:
References:
Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD). (12 December, 2022). How flu viruses can change: “drift” and “shift”. U.S. Department of Health & Human Services. https://www.cdc.gov/flu/about/viruses/change.htm
Couch, R. B. (1996). Orthomyxoviruses. Medical Microbiology. 4th edition. https://www.ncbi.nlm.nih.gov/books/NBK8611/
Klepeis, N. E., Nelson, W. C., Ott, W. R., Robinson, J. P., Tsang, A. M., Switzer, P., … & Engelmann, W. H. (2001). The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of Exposure Science & Environmental Epidemiology, 11(3), 231-252. https://www.nature.com/articles/7500165
Lowen, A. C., & Steel, J. (2014). Roles of humidity and temperature in shaping influenza seasonality. Journal of virology, 88(14), 7692 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4097773
Moriyama, M., Hugentobler, W. J., & Iwasaki, A. (2020). Seasonality of respiratory viral infections. Annual review of virology, 7, 83-101. https://www.annualreviews.org/doi/10.1146/annurev-virology-012420-022445
Scroll down to listen to this article.
I remember the early days of COVID-19, everything was new and scary and dangerous and no one knew what was going on. It seemed to be very dangerous for two groups of people, those of advanced age and those who were immunocompromised. Vaccines rolled out, testing became easy, and things have calmed down quite a bit. But corona isn’t all limes on the beach, as any virus, it’s still dangerous for people who are immunocompromised. What does this term mean, who are the immunocompromised, and is there anything they can do in the new reality of COVID-19?
Immunocompromised is a broad term. It indicates that a person’s immune system cannot generate an appropriate response to infection. When bacteria or viruses make it inside these people’s bodies, they cause much more damage and are very hard to control. The immune system is very, very complicated, so there are many ways a person can become immunocompromised. We can generally lump these into two categories: primary and secondary immunodeficiency.
Primary immunodeficiency means the condition is built-in to the body; it’s genetic. Primary immunodeficiencies are fairly rare, with <0.1% of the population experiencing them. The rest of the almost 3% of people who have immunodeficiencies have secondary immunodeficiencies.Infectious diseases (such as HIV), malnutrition, age, surgery, environmental stress, and immunosuppressive drugs can all cause secondary immunodeficiency. Immunodeficiency affects millions of Americans. Women are twice as likely as men to have immunodeficiency; it is most common in white Americans and those aged 50-59. Nearly 3% of the population – over 9 million Americans – are suspected to have immunodeficiency.
Unfortunately, immunodeficiency can greatly reduce a person’s ability to deal with a COVID-19 infection. The most obvious problem is that immunocompromised people are more susceptible to severe symptoms. A disproportionate amount of people who are hospitalized for COVID-19 are immunocompromised. Immunodeficiency doesn’t compromise, it packs a double-whammy. Those with a weak immune system also find vaccines less effective! In fact, 44% of people who had “breakthrough” cases (where they were vaccinated but still hospitalized) were immunocompromised. This is because the body is unable to produce enough protective antibodies for the body to be protected – a process called seroconversion.
Seroconversion is when antibodies are able to be detected in the blood. With vaccines, successful seroconversion indicates that the body is protected and has the equipment necessary to put up a good fight against the COVID-19 virus. When vaccinated against COVID-19, people with healthy immune systems showed seroconversion rates of 99%. The type of immunocompromisation affects how well vaccines produce seroconversion. People with solid tumor cancers, such as breast, colon, prostate, and lung cancer show seroconversion rates of 92%. Immune-inflammatory disorders like lupus, primary biliary cholangitis, psoriasis, and rheumatoid arthritis have seroconversion rates reduced to 78%. Vaccine effectiveness in people with blood cancers such as lymphomas, myeloma, and leukemia drops to 64%. Those with organ transplants have to be on strong immunosuppressive drugs to avoid organ rejection and because of this they have the lowest rates of seroconversion, only 27%.
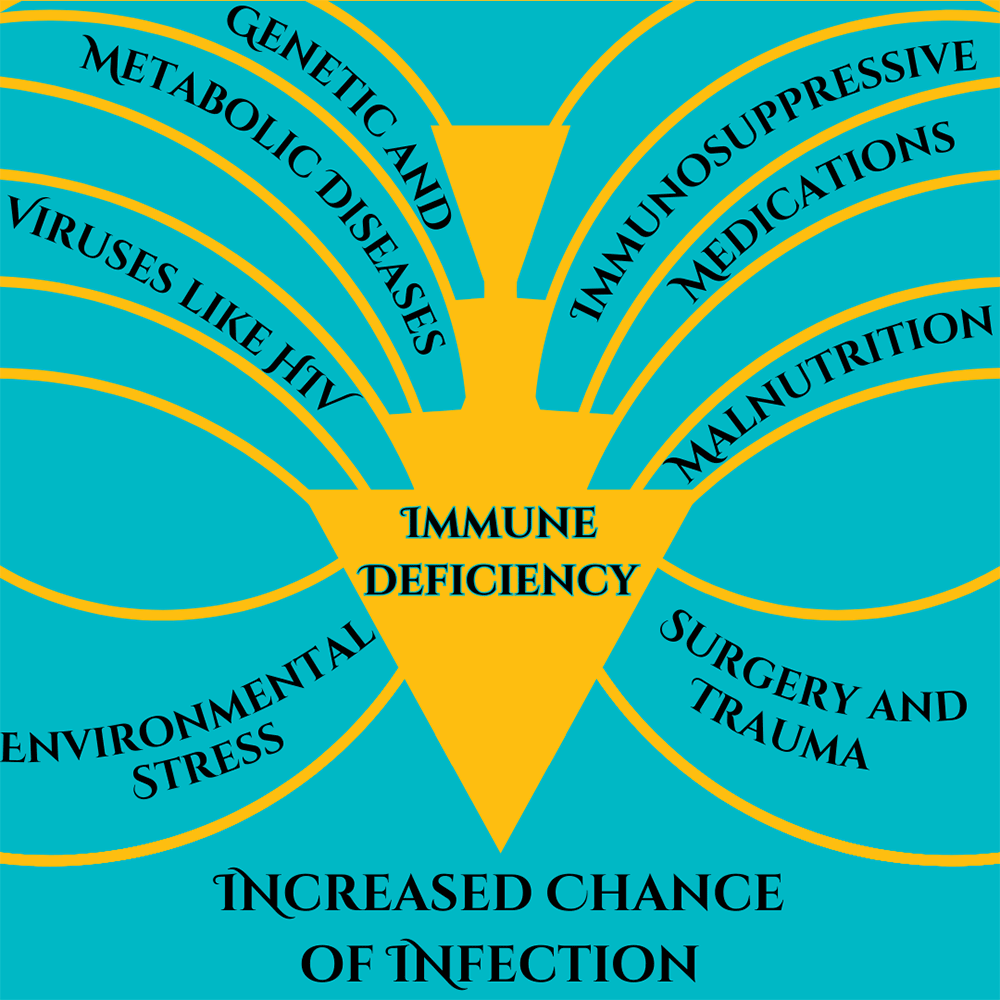
Some factors of immunocompromisation. Adapted from Chinen, J., & Shearer, W. T. (2010).
So what can immunocompromised people do to protect themselves against COVID-19? A lot of the same things as people who are immunocompetent! High quality masks and respirators can help. Avoiding crowds and indoor areas with poor ventilation is a must. Washing hands with soap and water is critical, though hand sanitizer is a good second option. Immunocompromised people who contract COVID-19 should contact their doctor or other healthcare provider right away. Isolating and using masks to prevent the spread is always a good idea. Immunocompromised people may also keep infections from getting out of control if their medical provider recommends an antiviral medication or convalescent plasma. Of course, the best way to avoid getting sick with COVID-19 is through prevention, including vaccines. A 27% seroconversion rate is much better than 0% after all. And there may be more hope for immunocompromised people, as new vaccines are being developed to serve this community.
Staff Writer / Editor Benton Lowey-Ball, BS, BFA
Listen to the article here:
References:
Boyle, J. M., & Buckley, R. H. (2007). Population prevalence of diagnosed primary immunodeficiency diseases in the United States. Journal of clinical immunology, 27, 497-502. https://link.springer.com/article/10.1007/s10875-007-9103-1
Chinen, J., & Shearer, W. T. (2010). Secondary immunodeficiencies, including HIV infection. Journal of Allergy and Clinical Immunology, 125(2), S195-S203. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6151868/
Harpaz, R., Dahl, R. M., & Dooling, K. L. (2016). Prevalence of immunosuppression among US adults, 2013. Jama, 316(23), 2547-2548. https://jamanetwork.com/journals/jama/fullarticle/2572798
National Institute of Health. (July 21, 2023). Special considerations in people who are immunocompromised. COVID-19 Treatment Guides, https://www.covid19treatmentguidelines.nih.gov/special-populations/immunocompromised/
Parker, E. P., Desai, S., Marti, M., Nohynek, H., Kaslow, D. C., Kochhar, S., … & Wilder-Smith, A. (2022). Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. The Lancet Global Health, 10(3), e326-e328. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(21)00593-3/fulltext
SY, L. A. W., SC, C. L. L., & Muthiah, L. M. (2021). Efficacy of COVID-19 vaccines in immunocompromised patients: A systematic review and meta-analysis. medRxiv. https://doi.org/10.1136/bmj-2021-068632
Scroll down to listen to this article.
I remember a joyous time when the world was young, and we had nothing to fear or worry about except climate change, an artificial intelligence cascade, political strife, and nuclear war. But we didn’t have to worry about COVID, so it was pretty idyllic. Now, over three years later, the biggest worries of COVID are winding down. The WHO declared COVID-19 to no longer be a global health emergency on May 5, 2023, and most places have lifted or lessened restrictions put in place to stem the spread. People have stopped wearing masks, and life seems to be back to normal. But not for everyone. Long COVID has been described as COVID symptoms that last longer than 5 weeks and may last months or longer. It affects around ⅓ of COVID patients, including over 85% of those that had to go to the hospital with severe symptoms. So what is long COVID, who gets it, how does it work, and is there anything to be done about it?
Long COVID, also called long haul COVID, post-COVID syndrome (PCS), or Post-acute sequelae of SARS-CoV-2 infection (PASC), is different from person to person but can be debilitating. During acute (normal) COVID infections, people experience trouble with breathing, joint pain, headache, fatigue, stomach problems, and loss of smell and taste. When I had COVID, my sense of taste was so poor that I started liking Limp Bizkit again. Long COVID symptoms are similar to those of acute COVID. Long-lasting and often crushing fatigue is the most common symptom. I’ve heard anecdotal stories of people who run out of energy just deciding what to eat during the day; it can be very intense. Other symptoms include muscle pain, cognitive impairment such as brain fog, headaches, anxiety, and more.
Around 140 million Americans have had COVID at some point. A recent study found that long COVID was more common and severe in people infected before the 2021 Omicron variant. Several other risk factors have also been identified. The severity of an acute COVID infection plays a role, as does having 5 or more separate symptoms. These can be mediated by being up to date on COVID vaccines. Long COVID is almost twice as common in women, and the risk is also increased if you are over 50 years old. Other health issues can also affect your chances. Being overweight, having psychiatric disorders, having asthma, and being in “poor general health” are risk factors. Interestingly, having latent Epstein-Barr virus might also increase your chances of developing long COVID.
So how does all of this work? In the acute stage of COVID, the virus spreads rapidly and tries to reproduce. It gains entry into cells using its spike protein to fool a receptor on our cells called ACE2. It then hijacks cell machinery to make copies fast. This may kill the infected cells but also brings in the immune system, which kills the invaders (if we’re lucky) and tends to cause some damage through inflammation. It is thought that long COVID occurs through many mechanisms. The immune system can be disrupted, other infections can take hold, we can experience chronic inflammation, and some body systems can be messed up. Even worse, sometimes organs can be damaged from the infection, and the virus might stick around for a while!
The major organs affected in long COVID can be deduced by looking at the symptoms. Trouble breathing, lung impairment, and low breath capacity may result from chronic inflammation and clotting in the lining of the lungs. Chest pain, irregular heartbeats, heart palpitations, and postural orthostatic tachycardia syndrome (POTS, low blood volume when standing up) are caused by chronic inflammation and cell death in the cardiovascular system. The cardiovascular system has an abundance of ACE2 receptors, meaning it is targeted for direct infection by the COVID virus. Fatigue, trouble sleeping, loss of taste and smell, and cognitive impairment are due to problems with our central nervous system. COVID can cross into the brain and cause inflammation of support cells and clotting (possibly leading to stroke!), and may also affect the brain stem. Nervous system problems affect around ⅓ of people by six months after COVID. There can also be problems with the kidney and pancreas.
So what can we do? Our best bet is to reduce the effects of COVID in the first place – or avoid it altogether. Staying up to date on COVID vaccines and boosters lowers both the severity of acute COVID and the risks of developing long COVID. Continuing to wear masks, washing hands frequently, and being careful around sick people can also help, and staying healthy with diet and exercise can give a leg up. Unfortunately, if you already have long COVID, there are no meds proven to cure it. Treating symptoms is our current best practice. Supplements may help, including B vitamins, iron, magnesium, zinc, and selenium. Multivitamins, mineral supplements, and probiotics have shown preliminary promise, as has the antiviral paxlovid. ANY alteration of medication, including supplements, should be run by your doctor first to ensure they are safe and don’t interact with other conditions or medications you may be on. Non-pharmaceutical solutions may also help. Physical rehabilitation – including pulmonary rehabilitation – as well as mental health and social assistance are vital to making it down the long road of long COVID.
Staff Writer / Editor Benton Lowey-Ball, BS, BFA
Listen to the article here:
References:
Conti, V., Corbi, G., Sabbatino, F., De Pascale, D., Sellitto, C., Stefanelli, B., … & Filippelli, A. (2023). Long COVID: clinical framing, biomarkers, and therapeutic approaches. Journal of Personalized Medicine, 13(2), 334. https://www.mdpi.com/2075-4426/13/2/334
Koc, H. C., Xiao, J., Liu, W., Li, Y., & Chen, G. (2022). Long COVID and its Management. International Journal of Biological Sciences, 18(12), 4768. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9305273/
Raveendran, A. V., Jayadevan, R., & Sashidharan, S. (2021). Long COVID: an overview. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 15(3), 869-875. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8056514/
Su, Y., Yuan, D., Chen, D. G., Ng, R. H., Wang, K., Choi, J., … & Heath, J. R. (2022). Multiple early factors anticipate post-acute COVID-19 sequelae. Cell, 185(5), 881-895. https://www.sciencedirect.com/science/article/pii/S0092867422000721
Thaweethai, T., Jolley, S. E., Karlson, E. W., Levitan, E. B., Levy, B., McComsey, G. A., … & Donohue, S. E. (2023). Development of a definition of postacute sequelae of SARS-CoV-2 infection. Jama. https://jamanetwork.com/journals/jama/fullarticle/2805540
Scroll down to listen to this article.
Have you ever been told not to share a drink with someone? A spoon? A toothbrush? I remember being told if I shared a drink, I might get mono. But what is mono, is it really so bad, what causes it, and what can we do about it?
To start, mono is not the real name. The disease is properly termed infectious mononucleosis. The term mononucleosis was first used in the 1920s to describe how some white blood cells, called lymphocytes, grow and have a central nucleus that resembles a different type of cell, a monocyte. Infectious mononucleosis has a few different causes, but 90% of cases are from a single virus; the Epstein-Barr virus (EBV). EBV is part of the herpes family of viruses. This category includes those that cause chickenpox/shingles and genital herpes, but each are their own separate type and can’t change into another.
EBV and infectious mononucleosis are very common. Per the NIH, 90% of people can expect to get EBV at some point in their lives. Most of the time we are infected as children and have few or no symptoms. It turns out those cootie shots weren’t working after all. The most common symptoms are very generic: fatigue, fever, sore throat, and swollen lymph nodes. These usually resolve within a few weeks. For some people, however, infectious mononucleosis will persist or lead to complications. These include a rash, liver enlargement, and spleen enlargement. Further issues may develop. The liver and spleen may have problems, including a ruptured spleen if the patient engages in intense physical activities. Additionally, EBV is one of the few viruses that can lead to the development of cancer. Even without severe complications, some cases of mono can last for several weeks.
So what is Epstein-Barr virus, and how does it cause problems? EBV is, as the name implies, a virus. These are “organisms at the edge of life” and need to infect host cells to replicate. EBV infects two types of cells, the epithelial cells that line the throat and B lymphocytes, a type of defensive white blood cell. When you are first infected, EBV is in a lytic phase. Lytic is from the Greek for “loosen,” and this stage is where EBV replicates rapidly and causes most of its symptoms. DNA inside the virus is open to being replicated and does so by the thousands and millions. EBV is sneaky, though, because not all of the virus particles do this. Instead, some of these particles bend their DNA into a circle inside of B cells. These B cells don’t “know” they’re infected and go about their business as usual. This is the latent stage. Latent EBV doesn’t reproduce on its own instead, it is copied when a cell splits. Latent EBV presents no symptoms and may even be integrated into our own DNA. Some will die with B cells during normal activity, but we can never be rid of EBV once we catch it. EBV occasionally reactivates and becomes lytic again. Scientists aren’t certain exactly why this happens but think it may be in response to a different infection, where the B cells are called into action. This can increase a person’s risk of developing nasopharyngeal cancer, certain lymphomas, or stomach cancers. Non-cancerous symptoms are caused by swollen, poorly performing B cells and infected cells that line the throat called epithelial cells.
So what can we do about infectious mononucleosis and EBV in general? Most of the time, we don’t need to do much. Most cases resolve on their own between 2-6 weeks as the body fights and EBV converts into the latent phase. During this time, treating symptoms at home can help: drink fluids, rest, and take over-the-counter medicine for pain and fever – acetaminophen is commonly used. Rest is very important, even if you don’t feel too fatigued. B cells are produced in the spleen, which can get swollen during infection. High-intensity activity, like sports, can cause it to rupture (not good). For more rare or difficult symptoms, your doctor may prescribe corticosteroids or antivirals. Antibiotics do not work, as EBV is not a bacteria. With potentially serious symptoms and EBV staying in your systems lifelong, prevention would be ideal, but is currently impractical due to the lack of a vaccine. There are also no medicines available to rid your body of EBV.
EBV is spread through saliva and other body fluids, so you can get it from sharing a straw, kissing, or getting an organ transplant. There is no approved vaccine against EBV yet, but we are hoping one may be available in the future. Look out for a clinical trial for EBV vaccines to help quench the kissing disease.
Staff Writer / Editor Benton Lowey-Ball, BS, BFA
Listen to the article here:
Sources:
U.S. Department of Health & Human Services/Centers for Disease Control and Prevention (September 28, 2020). About Epstein-Barr Virus https://www.cdc.gov/epstein-barr/about-ebv.html
Luzuriaga K, Sullivan JL. Infectious mononucleosis [published correction appears in N Engl J Med. 2010;363(15):1486]. N Engl J Med. 2010;362(21):1993-2000 https://www.researchgate.net/publication/44632890_Infectious_Mononucleosis
Odumade, O. A., Hogquist, K. A., & Balfour Jr, H. H. (2011). Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clinical microbiology reviews, 24(1), 193-209. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3021204/
Rybicki, E. (1990). The classification of organisms at the edge of life or problems with virus systematics. South African Journal of Science, 86(4), 182. https://journals.co.za/doi/pdf/10.10520/AJA00382353_6229
Listen to the article here:
A pandemic spread around the planet in the first quarter of the century. Not this century, however, but the last. The 1918 Flu Pandemic was the largest and deadliest outbreak of disease since the bubonic plague in the 1300s. The first official reported case was in Kansas in 1918. This gives the disease its proper name, the 1918 Influenza Pandemic. A much more common name, however, is the Spanish Flu.
The name Spanish Flu is an unfair name. Spain lost around a quarter million people to the 1918 Influenza. This is less than half as many as the USA, and fewer than Afghanistan, Mexico, Russia, Italy, and Japan. On top of that, India lost somewhere above 18 million people, and China between four and nine million. The big difference in losses was due to Spain being neutral during World War I. Because of this, they weren’t shy about publishing accurate data. Spain was the first country to publicly disclose that the pandemic was real, and other countries underreported or lied about numbers for years. This may strike some as familiar; during the COVID-19 pandemic, several national and local governments around the world tried to downplay the severity of COVID for political gain.
But what is influenza? Influenza, known as the flu, is very similar to COVID-19 in many ways. It is a viral infection, and its primary symptoms are cough, fever, joint pain, headache, body aches, and others. However, more serious complications such as pneumonia, liver damage, or brain problems can be triggered by influenza. It spreads through the air and can survive in water. Soap, changes in pH, and heat can destroy the influenza virus. The most dangerous part of influenza is how variable it is.
The influenza virus has several subtypes, and each of these mutates constantly. This makes it hard for the immune system to detect and fight new forms of the virus. It also means the specific symptoms of infections can change. In the 1918 influenza pandemic, the strain of influenza was particularly deadly for young, healthy people. This resulted in a lot of excess deaths compared to other strains.
The 1918 Influenza Pandemic was made much worse because of World War I. The war resulted in overcrowded barracks, troops stuffed in ships, and people crowding in shelters. Additionally, it spread wide and far as governments deployed troops around the world. A lack of accurate reporting and proactive measures certainly didn’t help. The biggest difference between then and now was medicine.
1918 was over a hundred years ago, but in the realm of medicine, it may as well have been much longer. Viruses were only discovered around 20 years prior, and there were no effective ways to fight them. There were no antiviral medications. For patients that developed pneumonia, there were no ventilators and no antibiotics. On top of this, there was no influenza vaccine.
The “Spanish flu” of 1918 helped refocus medical attention around pandemics – particularly influenza. In the early 1930s new vaccines were being developed from chicken eggs, and less than ten years later, the first experimental influenza vaccines were developed. Today, our yearly flu shots come from a direct line of response from the 1918 influenza pandemic. A century later, we have come a long way with medical advances, and since we know the influenza virus mutates regularly, the best way to help continue the fight against it is to participate in a clinical trial for the latest flu vaccines.
Written by Benton Lowey-Ball, BS Behavioral Neuroscience
Sources:
Hayden, F. G., & Palese, P. (2009). Influenza virus. Clinical virology, 943-976.
Jester, B., Uyeki, T. M., Jernigan, D. B., & Tumpey, T. M. (2019). Historical and clinical aspects of the 1918 H1N1 pandemic in the United States. Virology, 527, 32-37.
Johnson, N. P., & Mueller, J. (2002). Updating the accounts: global mortality of the 1918-1920″ Spanish” influenza pandemic. Bulletin of the History of Medicine, 105-115.
Knobler, S. L., Mack, A., Mahmoud, A., & Lemon, S. M. (2005). The threat of pandemic influenza: are we ready? workshop summary.
Mayer, J. (29 January 2019). “The Origin Of The Name ‘Spanish Flu’”. Science Friday. Retrieved 30 July 2021. https://www.sciencefriday.com/articles/the-origin-of-the-spanish-flu/
CDC, National Center for Immunization and Respiratory Diseases. (September 28, 2022). Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm
We’ve all heard enough about COVID-19, but it’s worth remembering that other viruses still try to get cozy in our respiratory system. One virus that is very prevalent in the United States is Respiratory Syncytial (“sin-sish-ul”) Virus, or RSV for short. It’s so widespread that the CDC states that nearly all children will get RSV before their second birthday. The oldest (above 65) and youngest (under 5) populations are most at risk of complications. Those most in danger are premature children, those with compromised immune systems, and those with underlying heart or lung diseases. All told, RSV accounts for around 177,000 hospitalizations of seniors (65+) and 58,000 children (under 5) each year.
RSV is easily transmissible. It passes from person to person through coughs, sneezes, or indirect means, like touching a doorknob and then your face. Most patients experience mild, cold-like symptoms. These include runny nose, fever, cough, sneezing, etc. Symptoms usually come in stages over a couple of weeks. Very young children and those at higher risk may experience more severe symptoms. In children under six, RSV might present as irritation, decreased activity, and breathing difficulty, which can be severe – and very scary! In adults over 65, severe symptoms can include a worsening of asthma or COPD, pneumonia, and the development of Congestive Heart Failure – a fluid buildup in the heart that prevents it from pumping effectively.
Much like the flu, RSV is seasonal. In most of the United States, the season is from September to February. The Florida Department of Health notes that Florida has a longer season than the rest of the nation. Here, the season for RSV is from August through April. The CDC has found that all across the south the year-round RSV cases increased. 2021 saw an unexpected surge of RSV over the summer. This is in part because the same tactics used to stem COVID-19 also protect against RSV. These protective measures include wearing masks, washing hands and surfaces, and social distancing. As these restrictions were lessened, cases of RSV rose to unprecedented summer levels.
Unfortunately, there is no cure for RSV. As it’s a virus, antibiotics are ineffective. Most patients will recover naturally. For others, best practices are treating symptoms by managing fever, pain, fluid intake, and any complications. For children and infants at severe risk, monthly Palivizumab injections may be available. Unfortunately, there are no publicly available vaccines for adults at increased risk. There are vaccines currently being researched that are going through clinical trials. With your help, we can find an effective RSV vaccine and help protect those at risk.
Written by: Benton Lowey-Ball, B.S. Behavioral Neuroscience
Sources:
Centers for Disease Control and Prevention. (2019). Respiratory Syncytial Virus Infection (RSV). Atlanta, USA.
Centers for Disease Control and Prevention. (2021). Increased interseasonal Respiratory Syncytial Virus (RSV) activity in parts of the southern United States. Atlanta, USA.
Florida Department of Health. (2022). Respiratory Syncytial Virus (RSV) in Florida. Tallahassee, USA
-
Novo Nordisk’s Wegovy (semaglutide) for weight loss
-
Biogen’s Aduhelm (aducanumab) for Alzheimer’s Disease
-
Pfizer’s PREVNAR 20 (pneumococcal 20-valent conjugate vaccine) for the prevention of pneumonia
https://www.nejm.org/doi/full/10.1056/nejmoa2102214
Novavax vaccine – Among a subgroup of HIV-negative participants, the vaccine was 60.1% efficacy against the B.1.351 South African variant.
You may have heard that people with diabetes are at a higher risk of contracting COVID-19. This is not the case. The truth is, people with diabetes are more likely to experience severe illness, long lasting effects, or even death if they are infected with COVID-19.
What We Know about Diabetes and COVID-19
In May, a nationwide multicentre observational study called the CORONADO study, observed the mortality risk in people with diabetes who were hospitalized for COVID-19. The study population was 88% type 2 diabetics and 12% type 1 diabetics. What they found was that one in ten diabetic patients hospitalized with COVID-19 died within seven days of hospital admission. One in five died within the first 28 days.
How Can We Improve These Numbers?
- Metformin – Recent studies have shown that metformin decreased the mortality rate of diabetic patients with COVID-19. Those who took metformin had an 11% mortality rate compared to 24% with type 2 diabetes who were not taking metformin when admitted to the hospital. These studies heavily indicate a strong, positive relationship between metformin, COVID and diabetes.
- Vaccine – another way to protect those battling diabetes from COVID-19 is to consider getting the vaccine. There have been three emergency use authorized vaccines: Pfizer, Moderna, and Johnson & Johnson. Each vaccine appears to be safe and effective in adults with diabetes. Rigorous clinical trials tested these vaccines for safety in adults of all ages, races and ethnicities and chronic health conditions.
-
-
-
-
-
- How will the vaccine affect blood sugar levels?
- Receiving the vaccine can cause symptoms of illness that can increase your glucose levels. However, if carefully monitored and correctly hydrated side effects can be minimal.
- Do diabetes medications affect the vaccine?
- Currently, there is no evidence to suggest that the COVID-19 vaccine will interact with current medications. However, it may be helpful to avoid injecting insulin or placing a glucose sensor near your vaccine injection site for several days after receiving the vaccine.
- Should I get vaccinated if I have diabetes and other health conditions?
- Complications of diabetes include heart disease and kidney disease. These conditions put one at higher risk or death from COVID-19.
- Vaccination should be a priority for patients with type 2 diabetes who are at very high risk of severe COVID-19 to help protect this vulnerable population.
- How will the vaccine affect blood sugar levels?
-
-
-
-
-
The flu is a respiratory infection caused by a group of viruses. Symptoms range from mild to severe and most commonly include body aches, cough, fever, headache and sore throat. The flu is contagious, spreading through tiny droplets from a cough, sneeze or even talking. We hear about it every year in the fall and winter because the viruses tend to survive longer in those seasons.
The flu vaccine is created to protect against influenza strains A and B. Once an individual is vaccinated, the body’s immune system responds by developing antibodies that will be ready to combat future infection. It takes about two weeks after a person has been vaccinated to gain protection. It is not unusual to briefly experience mild fatigue and muscle aches soon after injection as this represents an appropriate immune response, but because the ingredients in the flu shot have been inactivated, it is not possible to get “the flu” from the vaccine.
The flu vaccine is recommended for most people over six months of age and is given every year because:
The Circulating Flu Viruses Change
Influenza viruses undergo structural antigenic change and even mutation. Each February, flu experts gather and review the data to best decide what strains are predicted to circulate in the Northern Hemisphere during the upcoming flu season. Once the top 3 or 4 strains are identified, the viruses are grown then the vaccines manufactured using varying methods to create the safest and most effective flu shot. Typically, there is at least one and usually more than one new strain coverage included each year.
Immune Protection Declines Over Time
Over time, the antibodies created in response to that year’s vaccine begin to lose their effectiveness, though some individuals who received annual flu shots over many years maintain reserve immunity capable of preventing or softening the blow of a new infection even if challenged with a novel strain. The CDC recommends a yearly flu shot around October. Another advantage to getting the flu shot is that you are less able to carry and spread the virus to others that may have an altered immune status. Due to the fact older individuals don’t mount as robust of an immune response following vaccination, it is especially important for those over 65 years old to get the vaccine annually.
The CDC estimated that in the 2018-2019 flu season there were approximately 490,600 hospitalizations and 34,200 deaths from the flu. It’s safe to say the flu is a dangerous but preventable illness. We thank all volunteers that have contributed to now FDA approved and currently enrolling flu vaccine programs. Your participation has helped to save lives. Visit our enrolling studies page for more information as we work together to further develop the best prevention for this serious disease.
Source: Centers for Disease Control and Prevention
https://pubmed.ncbi.nlm.nih.gov/9360364/
https://www.cdc.gov/flu/prevent/keyfacts.htm
https://www.sciencedaily.com/releases/2019/03/190320110619.htm
There are five forms of antibodies that the human body makes. There are two forms that are relevant for COVID 19, Igm and IgG.
Igm is a big molecule, which is the first molecule that your body makes when you are exposed to a particular antigen or virus. This is an acute phase type of antibody.
IgG is a long-term antibody that has memory for your immune system and also protects you long-term. The actual length of long-term protection is not known.
Typically, when you have antibody testing, you are tested for both Igm and IgG. These tests are not perfect. If someone tests positive for Igm but not IgG, we’re not sure if they are protected.
If someone has no Igm antibodies and lots of IgG antibodies, they’re likely protected due to the long-term memory of IgG.
The length of time the antibodies remain detectable following an infection is not known.
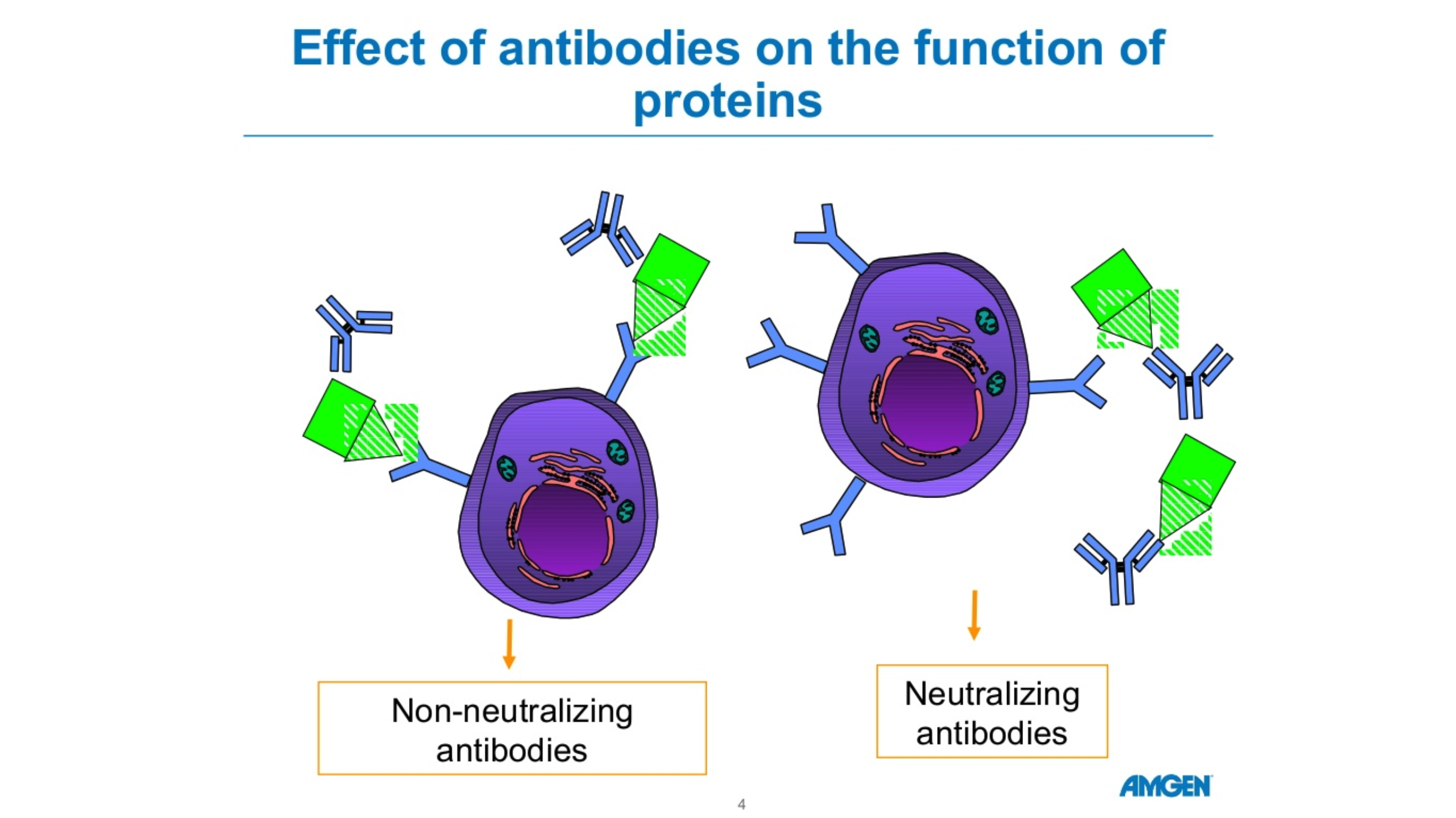
Source:
cdc.gov
Amgen Powerpoint
Our mission at ENCORE Research includes educating our community about health care news, particularly when standard media sources sensationalize the news. The coronavirus or COVID-19 story falls into this category. Patients and family members are asking, “How worried should we be?”
Our simple advice, “Don’t panic, but take sensible precautions.” Recent data, often reported incompletely, support the idea that we should think about COVID-19 as a bad strain of the flu.
New viruses are scary. Will these pathogens lead to minor nuisance illnesses like the common cold or horrible consequences like EBOLA, which kills nearly 90% of its victims? … or be more like the flu? Most people can understand and calibrate the severity of a virus based on their experience with the flu. Over the last decade, based on Center for Disease Control (CDC) statistics, the flu infects between 3-15% of all Americans each (mostly winter) season. Between 1 and 2% of flu victims require hospitalization and between 0.1 and 0.2% of victims die of complications of the illness. Death from the flu occurs mostly in infants, the very old and in folks with immune deficiency or other significant chronic illness.
For COVID-19, the initial reports of death rates of 4% in China and 10% in Iran now appear to reflect poor reporting (local officials and our media) and selective testing of patients. To make an accurate estimate of a death rate you need to know the total number of tests administered (which the media doesn’t typically report) to get a sense of whether all of the positive + tests are being captured. Otherwise, the death rate reflects what happens only to the sickest patients, those already on death’s door when they receive testing, rather than the full spectrum of disease.
As of this past weekend, we have good data to review to help us understand the true death rate of COVID-19. As of March 2, in South Korea, a hard hit country with a good healthcare system, the death rate is 0.51% (< 1%). South Korea has deployed extensive resources for testing of coronavirus. South Korea has now reported 22 deaths occurring among 4,335 patients infected by COVID-19 out more than 100,000 patients tested. The large number of tests and the relatively low number of positive tests helps us feel confident that South Korea has identified most patients with the illness, an essential part of the equation needed to determine the true death rate.
The South Korean death rate likely reflects a maximum rate of death. We suspect that the death rate will be lower in the US since we have had more warning and will intervene earlier with antiviral medications and support.
The low death rate is good news. Unfortunately, this good news has a down side. Because of the mild illness that results from infection in most folks who contract it, COVID-19 will likely spread extensively before it winds down. Ironically, there is a tradeoff between viral spread and lethality. The worst viruses, like EBOLA, kill most infected people, but do not spread widely because people get sick quickly and have far fewer contacts. With the flu or COVID-19, people may have minor symptoms that allow them to function in society and spread the virus. Governor DeSantis of Florida announced the first confirmed cases in the state on Sunday (March 1) and more cases will certainly occur, probably many more cases.
Another part of our mission at ENCORE involves helping to get new medical therapies to patients. We participate as an active research site in the medical product development system. We have already received contact about our ability to test a coronavirus vaccine in healthy patients wishing to avoid illness. These studies focus on prevention. We have assured the manufactures of the vaccines that we are poised and ready to jump in for the clinical study.
Many antiviral drugs “sit on the shelves” of pharmaceutical companies that have had proven efficacy against the SARS and MERS viruses – similar in structure but more deadly than COVID-19. We have no reason to believe that some of these drugs will not work against COVID-19, but they remain untested. At this time, since we do not have any patients with COVID-19 at our clinics, we will not participate in the treatment studies. However, if things change, we will respond.
So, what to do now?
If you would like to be on standby as a volunteer for a healthy patient vaccine study let us know. Contact – 904-730-0166 or Jaxresearch.com. In the meantime, wash your hands like crazy, keep hand sanitizer in the car and/or office, and use it at least 5 times a day during cold and flu season!
Michael J. Koren, MD, FACC, CPI
Chikungunya (chik·un·gun·ya) virus or CHIKV is an infection spread by a two types of Aedes mosquitoes, the yellow fever and Asian tiger species. These are the same mosquitoes that transmit Dengue and Zika virus. The name “chikungunya” derives from the Tanzanian word meaning “to become contorted”, and describes the stooped appearance of sufferers with joint pain. The virus is spread when a mosquito bites (feeds on) an infected individual then passes it on to a non-infected person on a subsequent bite. The Asian tiger mosquito has gradually become the dominant species in the US and is recognized for its ability to survive colder temperatures, therefore posing risk for infection spread into Florida and southeast USA. In 2019, Chikungunya virus infections were identified in 26 US states.
Symptoms:
Most patients who become infected develop high fever and joint pains within approximately a week. The severity varies but some patients experience debilitating aches which continue for years. The pain is caused by the immune system attacking itself causing inflammation of the tissue. Other symptoms of CHIKV viruses include:
- Headache
- Rash
- Muscle pain
- Pink eye
- Bent posture
Rare complications can occur. Infants and elderly adults are at highest risk for:
- Retinitis (inflammation of the retina in the eye which can cause permanent damage)
- Myocarditis (inflammation of the heart muscle which can lead to heart failure)
- Cranial nerve injury leading to facial pain, dizziness, hearing loss, facial twitch
Prevention:
Prevention methods include:
- Mosquito repellent (DEET, picaridin, or lemon eucalyptus applied to skin; permethrin applied to clothing)
- When practical, wear long sleeves and pants when exposed to Aedes mosquitoes
- When traveling to other countries, stay in places with air conditioning, window and door screens, netting
- Isolate the infected person from mosquitoes to prevent a fresh bite which can lead to spread to the next person
Treatments:
There is currently no antiviral therapy approved for Chikungunya. Treatments are focused on helping to relieve symptoms and spread.
Due to public health concerns over the potential for disease outbreak, the FDA granted “Fast Track” status in 2018 for development of the first effective and safe vaccine to prevent virus spread. You can help improve the future of medicine by participating in clinical trials. To learn more about participating in clinical research, visit our enrolling studies page or call us today!
References:
- https://www.who.int/news-room/fact-sheets/detail/chikungunya
- https://www.cdc.gov/chikungunya/hc/clinicalevaluation.html
- http://edis.ifas.ufl.edu/in696
- https://www.mosquito.org/page/repellents
A contagious virus that can cause infections in the lungs and respiratory tract.
You may have heard of the respiratory syncytial virus, in fact most people encounter RSV more than once, sometimes within the same year. Throughout older childhood and most of adulthood you may catch RSV during the winter and experience symptoms similar to the common cold. Symptoms range from mild to severe and include nasal congestion, cough, fever, wheezing, lethargy, and difficulty breathing.
What is so concerning about RSV?
It’s known that RSV shows severe symptoms in infants. However, recent studies have seen an increasing percentage of infected older adults with severe respiratory complications requiring hospitalization and occasional fatality.
I’ve had RSV before, so my immune system knows how to respond.
As we age, we encounter a natural degradation of our immune systems. While you may have encountered RSV in the past, infection after 65 years of age could entail severe respiratory complications as the immune system loses its ability to fight the virus. Studies show that RSV causes approximately 170,000 hospitalizations and around 14,000 deaths per year among older adults.
What can I do if I get infected?
There is currently no vaccine for the prevention of RSV, and because it’s a virus, antibiotics do not work. There are some treatments available, though usually pricey and used in extreme cases if you are already hospitalized.
The good news is there are several new preventative vaccines currently being developed. As an ENCORE Research community member, you have access to our cutting-edge research trials and are the first to know about new research. If you are interested in getting involved in any of our research studies, call your local office today!
Written by: Lana Borema