GRID VIEW
Diabetes is a widespread disease where the body cannot process sugars effectively. It has myriad effects on the body, including in the gut. Diabetes affects nearly every part of the gastrointestinal tract, from the throat to the anus. Diabetes is one of the most common causes of a condition called gastroparesis. Gastroparesis is from the Greek language. Gastro- means “stomach,” –paresis indicates a partial paralysis, so gastroparesis means a partial paralysis of the stomach; it empties food slowly. This condition is more likely to affect women than men, but there aren’t good numbers on how many people it affects in total. Symptoms of diabetes and gastroparesis may overlap but include feeling full, weight changes, nausea, vomiting, bloating, and diarrhea. Gastroparesis can lead to serious complications like malnutrition, decreased quality of life – including anxiety and depression, and significantly higher mortality. The effects of diabetes further exacerbate these complications. How diabetes actually affects stomach muscles is complex and fascinating. The digestive tract is lined with smooth muscle. Smooth muscle isn’t connected to bone and contracts in long, slow, low-energy waves. This is great for contracting blood vessels or moving food through the stomach and intestines. These are involuntary muscles, meaning we can’t consciously control them. In fact, the brain modulates the activity of gastrointestinal muscle, but it’s controlled by our “little brain” in the intestines! This little brain is made of around 500 million neurons and is called the enteric nervous system. The enteric nervous system is a distributed brain-like bunch of neurons and other cells spread throughout our abdomen that may be able to act independently of our big brain. It is responsible for getting the smooth muscle to contract rhythmically and in response to food. The enteric nervous system also includes cells that aren’t neurons. An important type is interstitial cells of Cajal (kuh-jaal). These are pacemaker cells that regularly generate electrical signals to coordinate muscle. The prominent players in the enteric nervous system, however, are neurons. Though neurons are electric, they communicate with other neurons by releasing neurotransmitters and receiving them in specialized receptors. These are often the same neurotransmitters we find in the brain, though they may have different functions. Dopamine is a great example. In the brain, dopamine receptors are part of the reward pathway, giving you feelings of pleasure and motivation. In the enteric nervous system, dopamine receptors act to suppress muscle movement. More dopamine in the brain makes you feel happy, but more dopamine in the gut restricts the movement of food. In diabetes, this can get all messed up. A key aspect of uncontrolled diabetes is the inability of the body to regulate blood sugar. This causes a few significant disturbances. Primary among these is neuron damage. Since the affected neurons are involuntary, we call this autonomic neuropathy. Autonomic neuropathy in the gastrointestinal tract can take the form of fewer neurons and ones that can’t communicate well because of damage. Diabetes can also lead to changes in the signaling pathways of smooth muscle and may destroy some of the pacemaking interstitial cells of Cajal. Together, these changes result in fewer smooth muscle contractions and a slower flow of food through the stomach and intestinal tract. The slow flow of food in gastroparesis can cause a feedback loop. Slow food is more likely to be mistaken for invaders, potentially causing inflammation. Even worse, the extra time in the digestive tract means there is more time for glucose, a major sugar, to get absorbed – increasing blood sugar and making the symptoms of diabetes worse. So, what can be done with diabetic gastroparesis? The first step is lifestyle alteration. Smaller and more frequent meals may help alleviate symptoms. Increasing noncarbonated liquids and decreasing fat and fiber may also help. Monitoring nutrient levels and electrolytes is critical. Beyond this, few medications are currently available. The most prominent are dopamine D2 receptor antagonists; meaning they inhibit the effect of dopamine. Metoclopramide (meh-tow-klow-pruh-mide) inhibits dopamine and mimics serotonin. It is effective at increasing the speed of emptying. It can cross the blood-brain barrier, however, causing unintended side effects in the brain. Domperidone (daam-peh-ruh-down) is another dopamine receptor antagonist. It doesn’t cross into the brain but can affect the heart, slowing the time it takes to recharge between beats. This medication is not approved in the United States, though it’s been approved in several other countries for decades. Clinical research is currently looking into new medications that may provide the same dopamine receptor inhibition as metoclopramide and domperidone but without the ability to affect the brain or heart! With the help of clinical volunteers, diabetic gastroparesis may pass quicker than we expected! At the time of writing this article, clinical research for diabetic gastroparesis is enrolling at our Nature Coast Clinical Research – Inverness office. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: Aswath, G. S., Foris, L. A., Ashwath, A. K., & Patel, K. (2017). Diabetic Gastroparesis. https://www.ncbi.nlm.nih.gov/books/NBK430794/ Bharucha, A. E., Kudva, Y. C., & Prichard, D. O. (2019). Diabetic gastroparesis. Endocrine reviews, 40(5), 1318-1352. https://academic.oup.com/edrv/article/40/5/1318/5487986?login=true Isola, S., Hussain, A., Dua, A., Singh, K., & Adams, N. (2018). Metoclopramide. https://www.ncbi.nlm.nih.gov/books/NBK519517/ Puoti, M. G., Assa, A., Benninga, M., Broekaert, I. J., Carpi, F. J. M., Saccomani, M. D., … & Borrelli, O. (2023). Drugs in focus: Domperidone. Journal of pediatric gastroenterology and nutrition, 77(2), e13-e22. DOI: 10.1097/MPG.0000000000003822 Sanders, K. M., Koh, S. D., Ro, S., & Ward, S. M. (2012). Regulation of gastrointestinal motility—insights from smooth muscle biology. Nature reviews Gastroenterology & hepatology, 9(11), 633-645. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4793911/ Sugumar, A., Singh, A., & Pasricha, P. J. (2008). A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clinical gastroenterology and hepatology, 6(7), 726-733. https://www.cghjournal.org/article/S1542-3565(08)00241-3/fulltext Tonini, M., Cipollina, L., Poluzzi, E., Crema, F., Corazza, G. R., & De Ponti, F. (2004). Clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Alimentary pharmacology & therapeutics, 19(4), 379-390. doi: 10.1111/j.1365-2036.2004.01867.x Uranga-Ocio, J. A., Bastús-Díez, S., Delkáder-Palacios, D., García-Cristóbal, N., Leal-García, M. Á., & Abalo, R. (2015). Enteric neuropathy associated to diabetes mellitus. https://digital.csic.es/handle/10261/241840 Yarandi, S. S., & Srinivasan, S. (2014). Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterology & Motility, 26(5), 611-624. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104990/
Listen to the article here:
We all do things for our parents. Some of us make them a card for their birthday, call them to say hello, or discover key mechanisms behind how the body metabolizes sugar in an attempt to save them from diabetes. Falling into that last category is the first American woman to win a Nobel Prize, Dr. Gerty Cori. She was born to a Jewish family in Prague in 1896. She became a doctor, got married, and worked in research at Carolinen Children’s Hospital. Gerty and her husband, Carl, collaborated and published multiple papers. Central Europe became challenging for Jewish people during the interwar period. She immigrated to the USA with her husband in the 1920s and naturalized in 1928. Her father was diagnosed with diabetes, and it’s said that’s where she got her inspiration. America in the first part of the 20th century was a tough place to be a female researcher. Gerty Cori’s husband was offered prestigious positions that were denied to Gerty based on her gender. Carl Cori would not stop collaborating with his wife and refused positions where Gerty was not welcome. They eventually were hired at Washington University in St. Louis, though Gerty was given a token salary 1/10th of what Carl made. There, Gerty and Carl collaborated and investigated how sugar was broken down and stored in the body. It was said that Gerty had the ideas, and together the husband and wife team made breakthroughs. This culminated in their description of how the body delivers energy to muscles during intense exercise in what came to be called the Cori Cycle. The Cori Cycle is essential to our understanding of how the liver and muscles work together. Our muscles need energy to do anything and everything. Normally, sugar in the form of glucose is broken down using oxygen, releasing energy. During prolonged and/or intense exercise, we can’t get enough oxygen to the muscles fast enough, and they have to produce energy without oxygen. To do this they convert glucose to pyruvate to lactate. Lactate is released into the blood, where it could cause damage if not for the liver. Gerty Cori and her husband’s experiments found that the liver regenerates glucose from the extra lactate. The liver uses more energy to make the glucose than the muscles can generate from it without oxygen, so the Cori Cycle is the shifting of the energy production from the muscles to the liver. Because of this and other critical work illuminating how the body metabolizes glucose, Gerty and her husband shared the 1947 Nobel Prize in Physiology and Medicine, making Gerty the first American woman to win the prize. Earlier that year, Gerty was also finally offered a full professorship at Washington University. She continued her work on glucose metabolism and spent time investigating enzymes and hormones. Her work would later be critical to our understanding of how glucose is regulated through the body, giving targets for diabetes medications. This Women’s History Month, as we honor the remarkable women who have impacted our world, let’s recognize the pioneering spirit of the first American woman to win a Nobel Prize in Physiology or Medicine. Her groundbreaking work laid the foundation for our understanding and treatment of diabetes. We can draw on her inspiration to make our parents proud and continue the legacy of progress and compassion. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: National Center for Biotechnology Information (2024). PubChem Pathway Summary for Pathway WP1946, Cori cycle, Source: WikiPathways. Retrieved March 19, 2024 from https://pubchem.ncbi.nlm.nih.gov/pathway/WikiPathways:WP1946. Washington University School of Medicine. (2004). Gerty Theresa Cori. Bernard Becker Medical Library. https://beckerexhibits.wustl.edu/mowihsp/bios/cori.htm Ginsberg, J. (2010). Carl and Gerty Cori and Carbohydrate Metabolism. National Historic Chemical Landmark. https://www.acs.org/education/whatischemistry/landmarks/carbohydratemetabolism.html Gerty Cori – Biographical. (1964). Nobel Lectures, Physiology or Medicine 1942-1962. Elsevier Publishing Company. https://www.nobelprize.org/prizes/medicine/1947/cori-gt/biographical/
Listen to the article here:
Type 2 Diabetes is a worldwide growing pandemic. Globally, around 425 million people have type 2 diabetes; that’s more than the entire US population. Almost 10% of Americans have type 2 diabetes, which is characterized by the body’s inability to regulate blood sugar (glucose). Uncontrolled high blood glucose levels can have severe long-term effects, impacting the cardiovascular system, brain function, and overall mortality. Because of the increased dangers associated with diabetes, there are a myriad of medications that target this disease. Unfortunately, these can be difficult to adhere to and usually require a daily activity like a pill, blood strip testing, and/or exercise. The more intensive and difficult these steps are (for instance, taking three pills a day vs one), the less likely people will be able to follow through. Additionally, though the medications can effectively reduce blood sugar levels and the risk of complications, they do not target the underlying disease. Medications may need to be increased or changed if the disease progresses. So, what is the underlying disease profile? The big picture causes of type 2 diabetes include genetics, diet, exercise, and overall weight. Inside the body, these risk factors and habits manifest as cellular changes. The most significant change that takes place is called insulin resistance. Insulin is an important hormone that helps your body manage blood sugar; when cells are resistant to insulin, they can’t adequately respond to high blood sugar. This is the key indicator of type 2 diabetes. Many medications attempt to correct insulin resistance by replicating or replacing chemicals involved in the insulin pathway – including insulin itself in advanced cases. These have been a significant boon to many patients, but what if we could go deeper? One of the changes many people see is in the intestines. The part of the intestines connected to the stomach is called the duodenum. The duodenum is short but important. It regulates the release of hormones like glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP), both disrupted in insulin resistance. Scientists have found evidence that people with type 2 diabetes have increased duodenum tissue; a process called hypertrophy. Tissue removal in these patients may positively affect type 2 diabetes. One of the most successful therapies for type 2 diabetes is bariatric (weight loss) surgery that bypasses some or all of the duodenum. These surgeries have been shown to have an immediate and long-lasting effect on people with diabetes. Studies have found that A1C levels, which measure blood sugar, are completely back to normal range in patients who have had these surgeries at a rate five times higher than people on medication alone. It is thought that by bypassing or removing the duodenum, the hypertrophic cells stop interfering with the insulin process, and insulin resistance decreases or is outright reversed! Also, since surgery is a one-time deal, the adherence problems of pills and other daily activities are reduced or eliminated. Unfortunately, surgery is invasive, intensive, painful, and somewhat risky (10-20% have complications). Additionally, removing parts of the duodenum can result in malabsorption of nutrients. To solve this, researchers have devised a new investigative procedure for tackling type 2 diabetes. Instead of cutting the body open and removing, adding, or rearranging the intestinal tract, a new approach called the Revita system is being tested. This system revolves around a catheter that moves into the duodenum and ablates, or removes, the top layer of hypertrophic duodenum tissue. Early studies have shown that by targeting just the hypertrophic tissue, the duodenum will heal and retain its function while blood sugar normalizes. This is an exciting potential alternative to normal bariatric surgery for people with type 2 diabetes. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: Cummings, D. E., Overduin, J., & Foster-Schubert, K. E. (2004). Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. The Journal of Clinical Endocrinology & Metabolism, 89(6), 2608-2615. https://academic.oup.com/jcem/article/89/6/2608/2870294 Jacobsen, S. H., Olesen, S. C., Dirksen, C., Jørgensen, N. B., Bojsen-Møller, K. N., Kielgast, U., … & Madsbad, S. (2012). Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obesity surgery, 22, 1084-1096. https://link.springer.com/article/10.1007/s11695-012-0621-4 Rubino, F., Forgione, A., Cummings, D. E., Vix, M., Gnuli, D., Mingrone, G., … & Marescaux, J. (2006). The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Annals of surgery, 244(5), 741. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1856597/ Rubino, F. (2008). Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes care, 31(Supplement_2), S290-S296. https://doi.org/10.2337/dc08-s271 Schauer, P. R., Bhatt, D. L., Kirwan, J. P., Wolski, K., Aminian, A., Brethauer, S. A., … & Kashyap, S. R. (2017). Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. New England Journal of Medicine, 376(7), 641-651. https://www.nejm.org/doi/full/10.1056/nejmoa1600869 Theodorakis, M. J., Carlson, O., Michopoulos, S., Doyle, M. E., Juhaszova, M., Petraki, K., & Egan, J. M. (2006). Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. American Journal of Physiology-Endocrinology and Metabolism, 290(3), E550-E559. https://journals.physiology.org/doi/full/10.1152/ajpendo.00326.2004 Wickremesekera, K., Miller, G., Naotunne, T. D., Knowles, G., & Stubbs, R. S. (2005). Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obesity surgery, 15(4), 474-481. https://link.springer.com/article/10.1381/0960892053723402 van Baar, A. C., Holleman, F., Crenier, L., Haidry, R., Magee, C., Hopkins, D., … & Bergman, J. J. (2020). Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut, 69(2), 295-303. https://gut.bmj.com/content/69/2/295.abstract
Scroll down to listen to this article.
Listen to the article here:
Sugars are sweet, tasty, and disastrous for your health in large quantities. They are also ubiquitous in modern society. We find them added to everything from salad dressings, drinks, bread, even peanut butter! Sugars and other carbohydrates make up over half of the calories consumed by Americans. Carbohydrates are broken down into a simple sugar called glucose and delivered around the body after eating. This can be surprisingly tricky. Too little glucose and cells can’t function. Too much and it gets converted to fat and damages the metabolic system, heart, and bloodstream. Type 1 Diabetes is a condition where the body doesn’t produce enough insulin. Type 2 Diabetes is more complicated; the body doesn’t respond to raised glucose in the bloodstream properly. A big culprit for failure is when insulin isn’t released well. To remedy this a class of drugs called glucagon-like peptide-1 (GLP-1) agonists have been developed. Agonist in this case means the medication has a similar function to the natural hormone. The opposite is an antagonist, which acts in opposition to them. GLP-1 medicines include Trulicity, Mounjaro, and semaglutide / Ozempic. In this article, we will review how insulin works, how GLP-1 works on the cellular level, and what GLP-1 medicines do to the body. Insulin is the main hormone that tells your body how to process sugar. Before we can understand how GLP-1 works, we need to understand the healthy release of insulin. This starts in the pancreas, an organ near our gut. The pancreas is filled with many types of cells called islet cells. These are responsible for regulating the balance of glucose in our bloodstream. Two major types are alpha and beta islet cells. Alpha islet cells produce glucagon and GLP-1. Glucagon tells the liver to increase blood sugar when you need energy. Beta cells make insulin and amylin, which help lower blood sugar. Alpha and beta islet cells work in opposition. They keep each other in check and our blood sugar levels just right. In Type 1 Diabetes, alpha cells may be dysfunctional and beta cells don’t exist or get destroyed. WIth Type 2 Diabetes, problems can occur when beta cells don’t function properly. Beta cells make insulin and release it in two stages. When these cells detect high blood glucose, they “trigger” and release insulin right away. This short response lasts 10-20 minutes, but is still several steps long. After triggering, a complicated “amplifying” pathway turns on to produce and release more insulin. Together this is powerful, slow, complex and has many potential points of failure. Beta cells are vital, and when they fail it often signals the transition from obesity to Type 2 Diabetes. GLP-1 is like a shortcut for beta cells. When it is detected the triggering response is primed and the cells are ready to release insulin as soon as glucose is detected. This pathway bypasses a lot of the complicated cellular machinery that is damaged in diabetic patients. The upshot is that GLP-1 stimulates insulin release from islet cells. An added benefit is that the insulin is only released in the presence of elevated glucose. This is good because you don’t release too much insulin, which can be dangerous. GLP-1 medications also last much longer in the body than natural GLP-1, giving longer-term effects which can last for up to a day! Now we know a little of how GLP-1 acts inside our cells, but what effects does this have on the body? Many, and widespread, it turns out! GLP-1 affects cells all over the body. The three biggest effects are decreased blood glucose, appetite suppression, and weight loss. Insulin decreases blood glucose, and GLP-1 increases the response to glucose. But GLP-1 medications have a secret extra benefit. Remember that alpha and beta cells work opposite each other. Normally when blood sugar is low, we release GLP-1 from our pancreas along with glucagon. Glucagon is very useful, and one of its uses is to stimulate the liver into producing more blood sugar. GLP-1 medications suppress glucagon production and the liver stays quiet. The pancreas still releases insulin, but the liver produces 45% less glucose! GLP-1 affects two of the biggest portions our appetite: our stomach and our brain. It slows the absorption of nutrients from the stomach, a process called gastroparesis. Food – and the glucose inside – is retained in the stomach and gut instead of the bloodstream. GLP-1 can also affect the brain. It can cross from the bloodstream into the brain, but also affect the vagus nerve – the major nerve connecting the brain and gut. Here it acts on the hypothalamus, suppressing the appetite and giving you feelings of being full. With the stomach slowing down and the brain signaling that it’s full, we tend to eat less. Combined, lower blood sugar and appetite can have serious effects on weight. This can be a big benefit of GLP-1 medications. Weight loss is linked with better outcomes for Type 2 Diabetes patients. Getting to a healthy weight is also good for the heart, joints, liver, and so on. Significant weight loss has been seen with GLP-1. Let’s not sugar-coat this though; not all weight loss is created equal. Ideally we’d cut our body fat while maintaining – or building – our muscle. This is especially true with diabetes, as skeletal muscle uses up extra glucose. Unfortunately, when we lose weight through diet restriction we lose more than just fat. This is true of gastric surgery, diet-induced weight loss, and GLP-1 medications. In GLP-1 medication studies, 20-50% of the weight lost is things other than fat – including muscle. Studies vary widely. The type of GLP-1 medication and other medications patients are taking may affect this. The best way to offset this is through building muscle with exercise! GLP-1 medications are truly amazing. They increase insulin response, lower blood glucose, suppress appetite, and lead to weight loss. It’s not all sugar and spice, however. Side effects can be rough, including vomiting and diarrhea. Additionally, meds can’t do it alone. When taking GLP-1 medications, the goal should still be to create an environment conducive to healthy living. Limiting carbohydrate intake is one critical step. Exercising is another. When fighting weight loss, victory is very sweet, but our diets shouldn’t be! Written By Benton Lowey-Ball, BS Behavioral Neuroscience
Sources: Campbell, J. E., & Newgard, C. B. (2021). Mechanisms controlling pancreatic islet cell function in insulin secretion. Nature reviews Molecular cell biology, 22(2), 142-158. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8115730/ Cervera, A., Wajcberg, E., Sriwijitkamol, A., Fernandez, M., Zuo, P., Triplitt, C., … & Cersosimo, E. (2008). Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. American Journal of Physiology-Endocrinology and Metabolism, 294(5), E846-E852.https://journals.physiology.org/doi/full/10.1152/ajpendo.00030.2008 Cohen, E., Cragg, M., deFonseka, J., Hite, A., Rosenberg, M., & Zhou, B. (2015). Statistical review of US macronutrient consumption data, 1965–2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition, 31(5), 727-732. https://pubmed.ncbi.nlm.nih.gov/25837220/ Drucker, D. J. (2018). Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell metabolism, 27(4), 740-756. https://www.sciencedirect.com/science/article/pii/S1550413118301797 Dungan, K., & DeSantis, A. (2013). Glucagon-like peptide-1-based therapies for the treatment of type 2 diabetes mellitus. https://www.uptodate.com/contents/glucagon-like-peptide-1-based-therapies-for-the-treatment-of-type-2-diabetes-mellitus#H1 Baggio, L. L., & Drucker, D. J. (2014). Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. The Journal of clinical investigation, 124(10), 4223-4226.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4191040/ Sargeant, J. A., Henson, J., King, J. A., Yates, T., Khunti, K., & Davies, M. J. (2019). A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinology and Metabolism, 34(3), 247-262. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6769337/
Listen to the article here:
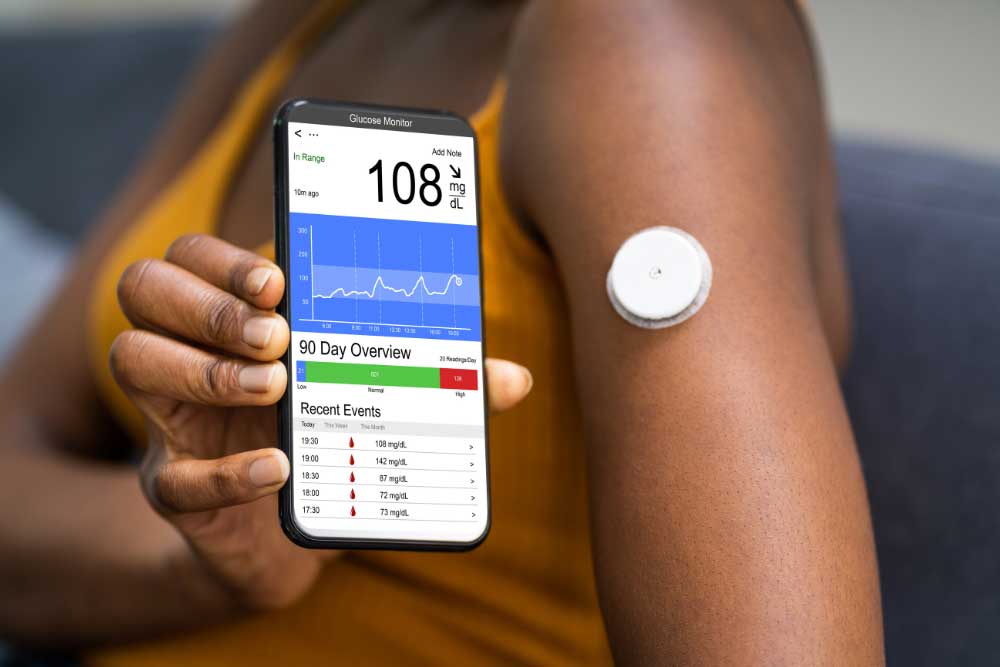
In fifth grade, I learned that mitochondria are the powerhouses of the cell. But what’s the fuel? The answer is carbohydrates. Big carbohydrates are broken down by digestion and converted into a couple of simple sugars. The most abundant of these simple sugars in our bodies is glucose. Glucose is small, simple, and packed with energy. We transport it through our bloodstream to cells in our body. Glucose levels are regulated by the liver and pancreas. Unfortunately, conditions like diabetes can result in the dysregulation of blood glucose levels. Having too much sugar in the blood is very bad over time. It can result in damage to the eyes, kidneys, nerves, and heart. On the flip side, having low blood sugar can get dangerous right away. Glucose is the fuel that powers our cells, without it the brain and other organs can’t function. We know that glucose is critical to body function. We also know that glucose levels can get out of control. What can we do to make sure glucose levels stay safe? The most important piece of the puzzle is information. Good information on what our blood glucose levels are is critical to know what to do. We get this information by testing our blood glucose levels. There are three major ways of testing blood glucose; chemical redox reactions, color change, and enzyme-based reactions. Now we know the chemical methods of measuring glucose, but how do we actually test our glucose level? Three broad testing types exist: oral, self-test, and continuous glucose monitors. These are differentiated mainly by the frequency and invasiveness of the test. As the old saying goes, knowledge is power! With the help of the latest CGM technology, we are able to see information in real-time such as how food, exercise, and stress impact glucose levels. This helps us take immediate action to manage our glucose levels. So, take action to keep your blood glucose in the healthy range with your new knowledge, a good diet, and consistent exercise. Make sure it stays there by monitoring your blood glucose levels regularly. Keep your eyes open to look for new studies looking at ways to monitor your blood glucose and keep your cells powered up!
Sources: American Diabetes Association (n.d.). Understanding A1C diagnosis. American Diabetes Association. https://diabetes.org/diabetes/a1c/diagnosis McMILLIN, J. M. (1990). Blood glucose. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Chapter 141. https://www.ncbi.nlm.nih.gov/books/NBK248/ Wang, H. C., & Lee, A. R. (2015). Recent developments in blood glucose sensors. Journal of food and drug analysis, 23(2), 191-200. https://doi.org/10.1016/j.jfda.2014.12.001
Listen to the article here:
How does the body use energy? After we eat, most food is broken down into parts that cells can use for energy. The bloodstream carries these pieces through the bloodstream to our cells, which let them in and convert food to energy. In some cases, the cells don’t let food particles in. In these cases, the problem may be diabetes. Cells need to separate their insides from the environment around them. Cells only let in specific molecules at specific times. Insulin is the molecule that tells cells to let in sugars in the form of glucose. It is produced by the pancreas and is released when the pancreas detects high levels of sugars in the blood. In some cases, such as with obesity, fatty acids can disrupt how cells absorb and use sugar in the blood. When this happens, cells are less sensitive to insulin and absorb less blood sugar per unit of insulin in the blood. Since blood sugar stays high, the pancreas produces more and more insulin, which has less and less effect. Cells can’t respond to all the excess insulin and are increasingly resistant to its effects. Insulin is also the hormone the pancreas uses to communicate with the liver about blood sugar. When the liver detects insulin it converts blood glucose into glycogen, a short term storage molecule. When high levels of insulin persist, the liver sends extra energy to fat cells. After long periods of insulin resistance, the pancreas itself stops working properly. Pancreatic cells become damaged and unable to produce insulin. This is called Type 2 Diabetes (T2D). With T2Ds, blood sugar stays high, insulin stops being produced, any produced insulin is less effective, and cells stop metabolizing properly. On top of this, the body gains excess weight which can stress the pancreas further. Other symptoms include cardiovascular disease, nerve dysfunction in the extremities (called neuropathy), and increased chance of death. Diabetes is very common in the United States. Tens of millions of Americans have T2D. Type 1 diabetes is an autoimmune disorder which results in pancreatic damage. Type 2 diabetes is an insulin resistance disorder and can have a slow onset. Major risk factors are obesity and lack of exercise. These should be the first steps to managing T2D as well. When a healthy diet and exercise aren’t enough to manage healthy blood sugars, or aren’t an option, several key medications exist to help with type 2 diabetes: Altogether, there are several medications which may be helpful for controlling type 2 diabetes. Discovering how these medications interact, lowering side effects, and finding treatments that are easy and straightforward is key. If you have type 2 diabetes, look for enrolling studies soon and improve your diet and exercise if possible! Written by Benton Lowey-Ball, BS Behavioral Neuroscience
Sources: Berg, J. M., Tymoczko, J. L., & Stryer, L. (2012). Biochemistry (7th Ed., pp 798-803). New York: W. H. Freeman and Company DeFronzo, R. A., Ferrannini, E., Groop, L., Henry, R. R., Herman, W. H., Holst, J. J., … & Weiss, R. (2015). Type 2 diabetes mellitus. Nature reviews Disease primers, 1(1), 1-22. https://www.nature.com/articles/nrdp201519 Olokoba, A. B., Obateru, O. A., & Olokoba, L. B. (2012). Type 2 diabetes mellitus: a review of current trends. Oman medical journal, 27(4), 269. http://doi.org/10.5001/omj.2012.68 Rena, G., Hardie, D. G., & Pearson, E. R. (2017). The mechanisms of action of metformin. Diabetologia, 60(9), 1577-1585. https://doi.org/10.1007%2Fs00125-017-4342-z U.S. Department of Health & Human Services/Centers for Disease Control and Prevention (August 10, 2021). Insulin Resistance and Diabetes https://www.cdc.gov/diabetes/basics/insulin-resistance.html U.S. Department of Health & Human Services/Centers for Disease Control and Prevention (December 16, 2021). Type 2 Diabetes https://www.cdc.gov/diabetes/basics/type2.html Witters, L. A. (2001). The blooming of the French lilac. The Journal of clinical investigation, 108(8), 1105-1107.https://doi.org/10.1172%2FJCI14178
Listen to the article here:
Diabetic peripheral neuropathy is a type of nerve damage associated with diabetes that most commonly affects the peripheries of the body (toes, feet, legs, hands, and arms). Symptoms can range from mild to severe and can be painful, debilitating, or even fatal. The most common neuropathy symptom people seek medical attention for is pain. Since diabetic neuropathy currently does not have a cure, the best thing people can do is treat the pain and make sure their blood sugar levels are well controlled.
Diabetic neuropathy symptoms are often worse at night. Symptoms include:
- Lack of sensation to pain or temperature in those areas
- A tingling, burning or needle pricking feeling
- Sharp pain or cramps
- Extreme sensitivity to touch
- Problems with balance and coordination
People who have had diabetes for at least 25 years have the highest rates of nerve damage. Neuropathies are also more common in people who cannot keep their blood sugar under control, have high blood pressure, or are obese.
Current treatment options for pain are limited by poor effectiveness and high rates of side effects, leaving many patients without adequate pain control. With chronic use, nonsteroidal anti-inflammatory drugs (NSAIDs) pose a potentially serious gastrointestinal and liver toxicity risk. Opioids are commonly prescribed for moderate-to-severe pain but are limited by safety and tolerability issues and have high abuse rates. Opioid-associated death rates have also increased over the past two decades.
Given the limited treatment options, combined with the risks and ineffectiveness of currently available treatments, developing new treatments is vital for better pain management and health outcomes. We are involved in many cutting-edge research trials at ENCORE Research Group, and some of our locations currently have research studies for painful diabetic peripheral neuropathy. To learn more about participating in our cutting-edge clinical trials, call our main office today! (904)-730-0166
Novo Nordisk’s Wegovy (semaglutide) for weight loss
Biogen’s Aduhelm (aducanumab) for Alzheimer’s Disease
Pfizer’s PREVNAR 20 (pneumococcal 20-valent conjugate vaccine) for the prevention of pneumonia
https://www.nejm.org/doi/full/10.1056/nejmoa2102214 Novavax vaccine – Among a subgroup of HIV-negative participants, the vaccine was 60.1% efficacy against the B.1.351 South African variant. https://www.nejm.org/doi/full/10.1056/NEJMoa2103055
You may have heard that people with diabetes are at a higher risk of contracting COVID-19. This is not the case. The truth is, people with diabetes are more likely to experience severe illness, long lasting effects, or even death if they are infected with COVID-19. What We Know about Diabetes and COVID-19 In May, a nationwide multicentre observational study called the CORONADO study, observed the mortality risk in people with diabetes who were hospitalized for COVID-19. The study population was 88% type 2 diabetics and 12% type 1 diabetics. What they found was that one in ten diabetic patients hospitalized with COVID-19 died within seven days of hospital admission. One in five died within the first 28 days. How Can We Improve These Numbers?
As I was perusing an ancient text I came across an excerpt on the disease known as diabetes. The earliest mention of the disease I could find was by a Greek physician called Aretaeus in the first century AD. Aretaeus identified diabetes and named it after its symptoms of thirst and sweet urine. When I researched the period I was astounded to find the diagnosing physicians were called “water tasters” and would either taste the urine or see if insects were attracted to it.
My research revealed that diabetes wasn’t well understood until the 20th century. It was then that researchers discovered insulin from the pancreas helped control blood sugar levels. A few decades later in 1961 the researchers at Ames Diagnostics created the first blood sugar monitor. This monitor was called Ames Reflectance Meter and was only available in doctor’s offices and hospitals due to its cost. Around this time a pioneering man named Richard Bernstein mounted a campaign to make blood sugar monitoring at home acceptable and easy. He was so passionate about the cause, at the age of 45 he went to medical school and became an endocrinologist. His efforts here led to millions of people being able to monitor their blood sugar at home. Now information about diabetes is growing exponentially. The thought that all of this diabetes research led from tasting urine to affordable blood sugar measuring is amazing!
At ENCORE research, we are passionate about advancing health care. Without research, we wouldn’t have these advances in diabetes management!
Many doctors prescribe metformin to diabetic patients. Doctors trust the drug, particularly since the landmark United Kingdom Prospective Study that showed that overweight Type 2 diabetics on metformin lived longer and suffered fewer heart attacks than those with the same blood glucose levels achieved using insulin. The history of metformin provides a good example of how an unusual herb can become a powerful treatment.
Metformin originates from the plant Galega officinalis or French lilac, goat’s plant or goat’s rue. This plant was fed to goats to improve milk production. It grows as a perennial herb, 3 feet tall, with purple, blue or white flowers and was used in the Middle Ages to treat frequent urination, a side effect of diabetes. The Native American Seminole tribe used the insecticide roterone, found in the roots of Galega officinalis, in fishing. Fish were stunned by roterone, and were much easier to catch. This plant has powerful properties and is now widely considered poisonous.
Metformin was first described by scientists in 1920. Chemists found that they could make the active compound from this plant, guanidine, more tolerable to be ingested by bonding two guanidines together forming a biguanide. This compound, which could lower blood sugar, was first synthesized in 1929. However, insulin had also been discovered during this time and became the more popular option for controlling blood sugar. Metformin was neglected and ignored until 1950 when metformin was used to treat influenza. Doctors noted metformin decreased glucose levels.
In 1958, Metformin was finally released in the United Kingdom as a treatment of lowering blood sugars. This drug was clinically developed and called Glucophage (“glucose eater”). Glucophage was released in the United States in 1995 and quickly became a popular medicine. Finally after half a century, it’s potential was realized. Metformin has now become the world’s most widely prescribed anti-diabetic agent and is universally recommended as the first therapy for Type 2 diabetes.
Metformin works by lowering the rate of hepatic glucose production, which is three times higher in people with Type 2 diabetes. Probably through reduction of insulin resistance, metformin can reduce cardiac risk factors and may decrease cardiac events. Since metformin does not cause hypoglycemia (low blood sugar), it can be safely utilized in a variety of diseases such as pre-diabetes, gestational diabetes, polycystic ovary disease, sleep apnea, osteoporosis and cancers.
Metformin’s has also been noted to have a positive effect on longevity due to its anti-cancer effect. Since having Type 2 diabetes is a major risk for developing cancer (pancreas, bladder, ovary, breast, prostate, colon and liver) patients with Type 2 diabetes have a lower risk of developing these cancers if they are utilizing metformin.
This very economical and widely available medication has been shown to safely treat multiple diseases in addition to Type 2 diabetes and to improve longevity in both the diabetic and non-diabetic patient. Metformin has come a long way from its humble “roots”. Fortunately, research rediscovered this powerful perennial herb. And now metformin has ascended to being the drug of first choice for patients with Type 2 diabetes.
The word “tsunami” describes a huge wave caused by an underground disturbance. Diabetes falls under that definition in our view. According to a study in Diabetes and Endocrinology, two out of five American adults may develop Type II Diabetes in their lifetime. About 95% of US diabetics are Type II. Type II diabetes occurs due to a condition of insulin resistance. Insulin, a hormone produced in the pancreas, does not have a sufficient effect on blood sugar levels, despite high levels of production in Type II diabetics. Fortunately, thanks to clinical research, surviving this diabetic tsunami should soon become easier. New research breakthroughs may lead diabetes to “dry land.”
For those currently battling diabetes, recent technological breakthroughs may make pricking your finger a thing of the past! Researchers at Arizona State University developed a method of testing glucose through saliva. To use, one simply licks a biosensor strip. The biosensing device then uses an electrochemical analysis to calculate blood glucose levels instantly. The company overseeing the development of this technology hopes to have it on the market in the next few years.
While tracking your blood sugar should become simpler, new ways of taking your diabetes medication may help keep diabetics afloat. Scientists have developed a “smart patch” that contains live pancreatic beta cells. A “smart patch” uses a system of tiny needles – each about the size of an eyelash, to detect high blood glucose levels and automatically inject insulin when needed. The live beta cells used in the newest versions of the smart patch can control rising blood glucose levels for roughly 10 hours at a time. Since the beta cells stay outside of the body on the patch, one’s body should not reject the cells. Current testing has been successful in mice studies, but the patch has yet to be tested on humans. Maybe one day we will seek volunteers for the “smart patch” at all of our seven research sites!
Another interesting diabetes finding comes from researchers at the Ottawa Hospital. Scientists turned from puzzled to amazed when they discovered a bacteria-killing protein in the pancreas. The bacterial-killing protein called cathelicidin antimicrobial peptide (CAMP) is produced by the same cells that produce insulin. Hoping to find a link between CAMP and diabetes, they injected diabetes-prone rats with CAMP. Not only did the rats have a healthier, more regenerated pancreas, but they had an increase in beneficial gut bacteria. CAMP could be a breakthrough in diabetes treatment.
Having diabetes is a big deal, and high sugar levels are themselves often less of a risk than other associated features of diabetes. In fact complications, such as high cholesterol levels and weight gain can be more problematic. High levels of cholesterol increase your risk of having a heart attack or stroke. Weight gain leads to higher blood sugar levels, high blood pressure and arthritis in weight bearing joints. We have several studies which target these complications. Many studies targeting cholesterol are enrolling right now at any of our sites, so if you have diabetes, heart disease, or even just high cholesterol give us a call and we can find the right fit for you! We also have a diabetes study enrolling with a medication that is said to cause weight loss at our Fleming Island, St. Johns, and Inverness offices. The future of diabetes management is looking bright! These research studies and new technologies coming down the pipeline may be just the life raft you’ll need to stay afloat in the diabetic tsunami.