GRID VIEW
Last week we talked about the actions of the Nazis, which resulted in the World Medical Association’s Declaration of Helsinki. The declaration provides an ethical guideline for physicians engaging in research involving humans. It has since become a requirement in most medical research. Unfortunately, in America, a study that began seven years prior to World War II undermined this ideal. It wouldn’t be until the 1970s that this study was exposed, and America was faced with its own dark practice of human research. In 1932, doctors at the Tuskegee Institute (Now called Tuskegee University) started a fundamentally unethical experiment. The road to this study winds from good intentions to simple, terrible means. The historical context behind the study involves the Julius Rosenwald Fund, a wholly honorable philanthropic endeavor. This fund built schools in the South, funded Booker T. Washington to attend the Tuskegee Institute, and worked with the U.S. Public Health Service to provide medical services to the poorest Black Americans in the South. One of these medical service endeavors was providing syphilis treatment. Part of this effort involved cataloging the rate of syphilis in several areas. The great depression and Julius Rosenwald’s death brought much of this to a halt, and the syphilis project ended in 1932. Other scientists wanted to pick up where the Julius Rosenwald Fund left off. These scientists believed that different races experienced diseases differently. The new study would observe the ravages of untreated syphilis in Black populations. They already had the groundwork built. They had a large number of men with untreated syphilis, a nearby hospital at Tuskegee Institute, a bank of goodwill built by Rosenwald, and a store of trust in medical professionals and the U.S. Public Health Service. The scientists successfully exploited all of these. They intentionally coerced and deceived 400 Black Americans into their study. The study had no protocol, patients had no informed consent, and one of the major endpoints was to wait until patients died and then deceive their loved ones into allowing an autopsy. This alone is terrible, but it was – unfortunately – much worse. After the end of World War II, an effective treatment for syphilis became widely available: penicillin. The researchers didn’t provide this to their patients and, in fact, actively thwarted its use in this population. They convinced hospitals, government agencies, and even the U.S. military that the torturous observation of sick and dying people was more important than their health and well-being. This continued for 40 years and only ended in 1972 after the experiment started getting public attention and press. The Tuskegee study ended almost a decade after the Declaration of Helsinki and prompted the passage of the National Research Act and the creation of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. A group of 11 people spent four years developing the Belmont Report. Whereas the Declaration of Helsinki outlines ethical standards to follow, the Belmont Report gives specific guidelines and actionable procedures for determining the legitimacy of research involving human participants. It was initially incorporated into federal law in 1981 as the Protection of Human Subjects. The Belmont Report first outlines the difference between medical practice and research. The goal of practice is normal therapy with a reasonable chance of success, while the goal of research is to test a hypothesis and answer a question. Whereas practice follows the needs of the patient, research follows well-defined, written protocols. The Belmont Report maintains the same three ethical principles in the Declaration of Helsinki – Respect for Persons, Beneficence, and Justice. It then outlines the practical, actionable processes: Informed Consent, Risk/Benefit Assessment, and Subject Selection. Respect for Persons is the idea that most people can make their own choices, and those that can’t must be afforded special protection. These protections must be assessed by third parties, protect participants from harm, and be periodically reevaluated. The Belmont Report applies respect for persons as Informed Consent. Informed consent requires that before a study begins, all information, including the procedure, risks, etc. be clearly written and organized. It requires comprehension by all potential human participants of the above information. Patients cannot be enrolled if they do not understand the informed consent, and the information can’t be written in tiny print and put off to the side like a used car ad. This also means that vulnerable populations (those with limited comprehension, like children) must have extra protection, and third parties (like parents) must help determine comprehension. Finally, informed consent is only given if it is voluntary. This means no large sums of money, threats, or exaggerated promises can be used to coerce people into studies. This can make it more difficult to enroll patients but is critical to avoiding disasters like the Tuskegee syphilis study. Beneficence is the obligation to secure the well-being of patients and to do no harm. Researchers may not injure some people to help others. This is applied as Risk/Benefit Analysis. Under this concept, all benefits must be weighed against potential risks. Risks are a possibility of harm, and include the chance harm will occur and the severity of the harm. This may include things like pain from the site of an injection and must be clearly laid out in the informed consent. Risk/Benefit Analysis is done by researchers, but is overseen and double-checked by Institutional Review Boards (IRBs) – third party boards that oversee studies involving human participants. The Belmont Report lays out specific ways these IRBs assess the risks of a study. These are then weighed against benefits. Benefits are a positive change in health or welfare and are usually much clearer – the alleviation of symptoms from being in a medical study, for instance. Justice is an equal distribution of burdens and benefits across society. Further, justice demands that the groups that participate in research should be the ones that receive the benefits. The racially biased attitudes that formed the basis of the Tuskegee study are the prime example of a failure of justice. These groups were denied benefits, and researchers demanded they take the burdens. The Belmont Report applies justice by way of Selection of Subjects. On the individual level this means that all subjects get fair treatment: you can’t give UF fans all of the investigational medication and FSU fans all placebo, for instance. Societally, the selection of subjects should be done in ways that minimize risks, like choosing adults before children. Selection should be done as fairly as possible and should target the populations that will benefit from research. Unfortunately, the Tuskegee study has undermined trust in government and research in some communities for the past 80 years. From this historical tragedy have arisen solid, unambiguous rules for conducting human research. These ensure safety, oversight, and mitigation of risk so participating in clinical research is beneficial for the community. With the Belmont Report, communities can get access to new medications, find relief from symptoms, and help define their legacy to find possible cures for future generations. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: National Commission for the Protection of Human Subject of Biomedical and Behavior Research. (1977). U.S. Department of Health and Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html Baker, S. M., Brawley, O. W., & Marks, L. S. (2005). Effects of untreated syphilis in the negro male, 1932 to 1972: a closure comes to the Tuskegee study, 2004. Urology, 65(6), 1259-1262. https://doi.org/10.1016/j.urology.2004.10.023 Brandt, A. M. (1978). Racism and research: the case of the Tuskegee Syphilis Study. Hastings center report, 21-29. https://doi.org/10.2307/3561468 Gray, F. D. (1998). The Tuskegee syphilis study: The real story and beyond. NewSouth Books.
Listen to the article here:
In 1945-1946, after the conclusion of the Second World War, several Nazi German leaders and doctors stood trial for crimes against humanity, war crimes, and other atrocious crimes. One of the trials, the so-called Doctor’s Trial (U.S.A. v. Karl Brandt et al.), helped shape how we view and perform clinical research today. One of the major defenses in the Doctor’s Trial was a lack of international law or agreement against the horrible activities the Nazis were doing. To remedy this, the trial ruling (in which seven defendants were sentenced to death) gave an outline of “Permissible Medical Experiments.” This was called the Nuremberg Code and was later expanded by the World Medical Association into the Declaration of Helsinki. The Declaration of Helsinki, originally released in 1964, is the cornerstone of modern clinical research. The medical atrocities during the Holocaust showed that the unsaid rules of medicine needed to be said, printed, and widely distributed. The World Medical Association’s original Declaration of Helsinki has been revised seven times and has since grown by 300%. It concerns the ethical treatment of human participants in medical research. The foundational notions of the Declaration of Helsinki rest on the following two ideas: “The health of my patient will be my first consideration” and “A physician shall act in the patient’s best interest when providing medical care.” The fact that these were up for debate is wild to me, but thanks to the Declaration of Helsinki these are now universal statements. The rest of the Declaration of Helsinki has similar “this should be obvious” content but has been critical for ensuring the safe and ethical treatment of people in research. The document first states that human medicine must be tried in humans at some point. There are also stipulations about what must happen before a study can begin. A protocol must be written that outlines the entire study. An independent review board (IRB) must approve the protocol and any changes. Funding and results should be transparent and public. The document also outlines in broad terms how to do this with respect, beneficence, and justice. Respect comes from the assertion that individual people matter more than new knowledge. This is how we get concepts like informed consent – that all trial participants must have full knowledge of what they are getting into before signing up. Respect also states that patients must voluntarily sign up and be able to discontinue at any time. Further, special protections must be in place for vulnerable populations – like children, prisoners, and people with mentally disabilities. Beneficence is the concept of weighing benefits against risks. In medical research, the benefits must always outweigh the risks. Benefits must help the individual person, a population, or society. Risks must be minimized wherever possible. This is one reason you often see a long list of things that can exclude a person (like being pregnant) from a study: to minimize risks. Beneficence also mandates that studies must be stopped if the risks become too high – if an unintended side effect emerges, for instance. Justice is the concept that research should be for the benefit of everyone. Medications that would target a specific population should be researched in that population. If left-handed people made up 90% of smokers, for instance, then smoking cessation studies should aim to enroll mostly left-handed people. Justice also means groups should be fairly selected and that studies should help people who live where the studies take place. You shouldn’t test a pants-lengthening drug on people in Bermuda (for their Bermuda shorts) and refuse to sell it to them. Altogether, these seem like common-sense rules to have in place, but they need to be written. The Nazis claimed the ends justify the means – even though their end goals were abhorrent. In this world, however, there are no ends – only means. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: Bošnjak, S. (2001). The declaration of Helsinki: The cornerstone of research ethics. Archive of Oncology, 9(3), 179-184. Nuremberg Trials Project (n.d.). NMT case 1. Harvard Law School. https://nuremberg.law.harvard.edu/nmt_1_intro Shrestha, B., & Dunn, L. (2019). The Declaration of Helsinki on medical research involving human subjects: a review of seventh revision. https://elibrary.nhrc.gov.np/handle/20.500.14356/1367 Taylor, T. (1955). Nuremberg Trials, The. Colum. L. Rev., 55, 488. https://www.jstor.org/stable/1119814?read-now=1&seq=38 United States Holocaust Memorial Museum, Washington, DC. (n.d.). The Nuremberg code. Holocaust Encyclopedia. Accessed 5/13/2024. https://encyclopedia.ushmm.org/content/en/article/the-nuremberg-code World Medical Association. (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama, 310(20), 2191-2194. https://jamanetwork.com/journals/jama/fullarticle/1760318/
Listen to the article here:
It’s spring, which means lots of sneezing, sweets, and sunlight! Of those three, sunlight is probably the healthiest, so let’s shine a light on what sunlight does in the body and why we need it. First, the sun is vital for keeping the planet habitable. Without the sun, we would all die very fast as oxygen would solidify on the surface, and we’d be unable to breathe. Beyond being vital to life, sunlight is used by the skin to produce Vitamin D! Vitamin D is actually a collection of very similar molecules called calciferols. These are fat-soluble steroid hormones that are used throughout the body. Vitamin D deficiency is a worldwide problem, and affects at least ⅓ of Americans. It is linked to complications in bones, kidneys, heart, and brain, as well as to diabetes, immune system issues, obesity, and poor pregnancy outcomes. Though indications of this deficiency seem robust, the solutions are anything but. Unfortunately, the effects of supplemental vitamin D, and therefore sunlight, are grossly understudied. Trial after trial after trial (check the extensive references list) shows that supplemental vitamin D – and in some cases supplemental light – does not have a significant effect on measurable outcomes for patients. These trials consistently find that the levels of circulating vitamin D in the bloodstream are increased, but symptoms are unaffected. The only results I could find from randomized, placebo-controlled clinical trials showing actual benefits to patients were for those with sickle-cell disease and in reducing respiratory infections in elderly patients. This is counter to “common knowledge” and to the assumed knowledge found in several research papers. Observational studies, where scientists look at populations, find a myriad of problems associated with vitamin D and sunlight deficiency. Let’s try to illuminate why clinical trials haven’t found benefits when giving supplemental vitamin D. The answer is likely that the problems that cause vitamin D deficiency aren’t solved by supplementation! Vitamin D production starts in the skin, then goes to the liver and kidneys before the body can use it. The symptoms associated with low levels of vitamin D may not improve if there are underlying liver or kidney issues, though those conditions can hinder the production of Vitamin D. Further, observational studies look at a population and investigate the correlations between items. This can tell us things that may be associated with each other but does not indicate that one thing is causing the other. An example would be looking at the availability of toilet paper and used car prices over the last 10 years. In 2020 toilet paper was unavailable and used car prices soared! This wasn’t because we needed toilet paper to run our used cars or anything; there was a pandemic disrupting supply chains! In the case of vitamin D deficiency, the lack of vitamin D is probably the effect of not going outside! If a mental disorder like depression keeps people indoors, this would lower the vitamin D they produce, not the other way around. On top of this, it is very difficult for scientists to isolate sunlight (and therefore vitamin D) from exercise. People who stay inside and never see the sun are, on average, less active. This can lead to some of the problems we associate with vitamin D deficiency, including bone issues, obesity, diabetes, and heart problems. In these cases vitamin D deficiency is more a canary in the coal mine than the smoke of a fire. So, what can we take away from this? First and most importantly is that in all of these clinical trials supplemental vitamin D has been found to be safe. If vitamin D helps you with an issue, there is nothing wrong with continuing your care. Always consult with your doctor before making changes to your medication. Second, clinical trials are vital to our medical system! Observational studies are no substitute for actual experimentation in a placebo-controlled, randomized trial. The common knowledge is that sunlight and vitamin D are good for us. This is true, but it’s best to start at the source, the sun (if you can), rather than supplementing after the fact. We should spend more time outside in the nice weather, but remember your sunscreen. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: Aranow, C., Kamen, D. L., Dall’Era, M., Massarotti, E. M., Mackay, M. C., Koumpouras, F., … & Diamond, B. (2015). Randomized, double‐blind, placebo‐controlled trial of the effect of vitamin D3 on the interferon signature in patients with systemic lupus erythematosus. Arthritis & rheumatology, 67(7), 1848-1857. https://doi.org/10.1002/art.39108 Burns, A. C., Saxena, R., Vetter, C., Phillips, A. J., Lane, J. M., & Cain, S. W. (2021). Time spent in outdoor light is associated with mood, sleep, and circadian rhythm-related outcomes: a cross-sectional and longitudinal study in over 400,000 UK Biobank participants. Journal of affective disorders, 295, 347-352. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8892387/ Eckard, A. R., O’Riordan, M. A., Rosebush, J. C., Lee, S. T., Habib, J. G., Ruff, J. H., … & McComsey, G. A. (2018). Vitamin D supplementation decreases immune activation and exhaustion in HIV-1-infected youth. Antiviral therapy, 23(4), 315-324. https://doi.org/10.3851/imp3199 Guralnik, J. M., Sternberg, A. L., Mitchell, C. M., Blackford, A. L., Schrack, J., Wanigatunga, A. A., … & STURDY Collaborative Research Group. (2022). Effects of vitamin D on physical function: results from the STURDY trial. The Journals of Gerontology: Series A, 77(8), 1585-1592. https://doi.org/10.1093/gerona/glab379 Ginde, A. A., Blatchford, P., Breese, K., Zarrabi, L., Linnebur, S. A., Wallace, J. I., & Schwartz, R. S. (2017). High‐dose monthly vitamin D for prevention of acute respiratory infection in older long‐term care residents: a randomized clinical trial. Journal of the American Geriatrics Society, 65(3), 496-503. https://pubmed.ncbi.nlm.nih.gov/27861708/ Hansen, K. E., Johnson, R. E., Chambers, K. R., Johnson, M. G., Lemon, C. C., Vo, T. N., & Marvdashti, S. (2015). Treatment of Vitamin D Insufficiency in Postmenopausal Women: A Randomized Clinical Trial. JAMA internal medicine, 175(10), 1612–1621. https://doi.org/10.1001/jamainternmed.2015.3874 Huiberts, L. M., & Smolders, K. C. (2021). Effects of vitamin D on mood and sleep in the healthy population: Interpretations from the serotonergic pathway. Sleep Medicine Reviews, 55, 101379. https://www.sciencedirect.com/science/article/pii/S1087079220301222 Javed, A., Kullo, I. J., Balagopal, P. B., & Kumar, S. (2016). Effect of vitamin D3 treatment on endothelial function in obese adolescents. Pediatric obesity, 11(4), 279-284. https://onlinelibrary.wiley.com/doi/10.1111/ijpo.12059 Juraschek, S. P., Miller III, E. R., Wanigatunga, A. A., Schrack, J. A., Michos, E. D., Mitchell, C. M., … & Appel, L. J. (2022). Effects of vitamin D supplementation on orthostatic hypotension: results from the STURDY trial. American journal of hypertension, 35(2), 192-199. https://doi.org/10.1093/ajh/hpab147 Karsy, M., Guan, J., Eli, I., Brock, A. A., Menacho, S. T., & Park, M. S. (2019). The effect of supplementation of vitamin D in neurocritical care patients: RandomizEd Clinical TrIal oF hYpovitaminosis D (RECTIFY). Journal of neurosurgery, 1–10. Advance online publication. https://doi.org/10.3171/2018.11.JNS182713 Michos, E. D., Kalyani, R. R., Blackford, A. L., Sternberg, A. L., Mitchell, C. M., Juraschek, S. P., … & Appel, L. J. (2022). The relationship of falls with achieved 25-Hydroxyvitamin D levels from vitamin D supplementation: the STURDY trial. Journal of the Endocrine Society, 6(6), bvac065. https://doi.org/10.1210/jendso/bvac065 Okereke, O. I., Reynolds, C. F., Mischoulon, D., Chang, G., Vyas, C. M., Cook, N. R., … & Manson, J. E. (2020). Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. Jama, 324(5), 471-480. https://jamanetwork.com/journals/jama/fullarticle/2768978 Osunkwo, I., Ziegler, T. R., Alvarez, J., McCracken, C., Cherry, K., Osunkwo, C. E., … & Tangpricha, V. (2012). High dose vitamin D therapy for chronic pain in children and adolescents with sickle cell disease: results of a randomized double blind pilot study. British journal of haematology, 159(2), 211-215. https://onlinelibrary.wiley.com/doi/abs/10.1111/bjh.12019 Øverland, S., Woicik, W., Sikora, L., Whittaker, K., Heli, H., Skjelkvåle, F. S., … & Colman, I. (2020). Seasonality and symptoms of depression: A systematic review of the literature. Epidemiology and psychiatric sciences, 29, e31. https://www.cambridge.org/core/journals/epidemiology-and-psychiatric-sciences/article/seasonality-and-symptoms-of-depression-a-systematic-review-of-the-literature/375F49B0149E903EFBDEDF9D53431B15 Palacios, C., & Gonzalez, L. (2014). Is vitamin D deficiency a major global public health problem?. The Journal of steroid biochemistry and molecular biology, 144, 138-145. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4018438/ Ponda, M. P., Liang, Y., Kim, J., Hutt, R., Dowd, K., Gilleaudeau, P., Sullivan-Whalen, M. M., Rodrick, T., Kim, D. J., Barash, I., Lowes, M. A., & Breslow, J. L. (2017). A randomized clinical trial in vitamin D-deficient adults comparing replenishment with oral vitamin D3 with narrow-band UV type B light: effects on cholesterol and the transcriptional profiles of skin and blood. The American journal of clinical nutrition, 105(5), 1230–1238. https://doi.org/10.3945/ajcn.116.150367 Rorie, A., Goldner, W. S., Lyden, E., & Poole, J. A. (2014). Beneficial role for supplemental vitamin D3 treatment in chronic urticaria: áaárandomized study. Annals of allergy, asthma & immunology, 112(4), 376-382. https://www.annallergy.org/article/S1081-1206(14)00012-X/abstract Sadock, B.J., Sadock, V.A., & Ruiz, P. (2017). Comprehensive textbook of psychiatry (Vol. 1/2). Sadock, B.J., Sadock, V.A., & Ruiz, P. (Eds.). Philadelphia: Lippincott Williams & Wilkins. Sokol, S. I., Srinivas, V., Crandall, J. P., Kim, M., Tellides, G., Lebastchi, A., … & Alderman, M. H. (2012). The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vascular medicine, 17(6), 394-404. https://journals.sagepub.com/doi/10.1177/1358863X12466709 Shaffer, J. A., Edmondson, D., Wasson, L. T., Falzon, L., Homma, K., Ezeokoli, N., … & Davidson, K. W. (2014). Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials. Psychosomatic medicine, 76(3), 190-196. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4008710/ Simha, V., Mahmood, M., Ansari, M., Spellman, C. W., & Shah, P. (2012). Effect of vitamin D replacement on insulin sensitivity in subjects with vitamin D deficiency. Journal of investigative medicine, 60(8), 1214-1218. https://doi.org/10.2310/jim.0b013e3182747c06 Sokol, S. I., Srinivas, V., Crandall, J. P., Kim, M., Tellides, G., Lebastchi, A., … & Alderman, M. H. (2012). The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vascular medicine, 17(6), 394-404. https://doi.org/10.1177/1358863×12466709 Wei, W., Shary, J. R., Garrett-Mayer, E., Anderson, B., Forestieri, N. E., Hollis, B. W., & Wagner, C. L. (2017). Bone mineral density during pregnancy in women participating in a randomized controlled trial of vitamin D supplementation. The American Journal of Clinical Nutrition, 106(6), 1422-1430. https://doi.org/10.3945/ajcn.116.140459 Winthorst, W. H., Bos, E. H., Roest, A. M., & de Jonge, P. (2020). Seasonality of mood and affect in a large general population sample. Plos one, 15(9), e0239033. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0239033 Wirz-Justice, A., Skene, D. J., & Münch, M. (2021). The relevance of daylight for humans. Biochemical pharmacology, 191, 114304. https://www.sciencedirect.com/science/article/pii/S0006295220305402 Zahoor, I., & Haq, E. (2017). Vitamin D and multiple sclerosis: An update. Exon Publications, 71-84. https://www.ncbi.nlm.nih.gov/books/NBK470154/
Listen to the article here:
This year has been exciting for clinical research. Our ENCORE sites completed 65 clinical trials (studies), and the FDA approved 66 new medications and vaccines nationwide. There is a long time between when a research organization or site, such as ours, completes a study and when a medication is ready for FDA review. Out of the 66 new medications or vaccines that were approved this year, ENCORE sites conducted seven of those clinical trials. That means all of you fantastic research volunteers helped get seven new medications or vaccines to market! In this article, we’ll review this year’s approvals and what they mean. Three new vaccines that received FDA approval this year had trials at ENCORE sites. Arexvy and Abrysvo were both approved for the treatment of Respiratory Syncytial Virus (RSV) in adults. This disease hospitalizes 177 thousand adults over 65 each year and causes over ten thousand deaths. Arexvy was the first vaccine approved against RSV in adults, and Abrysvo is intended for both prevention and treatment of RSV and is approved for use in pregnant women. Arexvy was studied at the Westside Center for Clinical Research and Abrysvo at Nature Coast Clinical Research – Crystal River. Ixchiq is the world’s first vaccine to be approved for Chikungunya. Chikungunya is spread to people by infected mosquitoes and causes fevers and joint pain. St. Johns Center for Clinical Research participated in two phase III clinical trials for Ixchiq. Two more ENCORE-researched medications approved this year were the first of their kind. Vowst has become the first medication to become FDA-approved for the treatment of C. difficile, also known as C. diff. C. difficile is a major health threat and causes colon inflammation. ENCORE Borland Groover Clinical Research investigated Vowst in the ECOSPOR trials. Veozah (fezolinetant) is the first non-hormonal treatment for moderate to severe vasomotor symptoms in women with menopause. These symptoms include hot flashes and night sweats, and Veozah targets the temperature centers of the brain to help with these symptoms. Three ENCORE research sites participated in the SKYLIGHT trials to investigate Veozah, Fleming Island Center for Clinical Research, St. Johns Center for Clinical Research, and Nature Coast Clinical Research – Crystal River. Two medications were approved, which may help with established diseases. Rinvoq (upadacitinib) is a daily pill for those with Crohn’s disease, an autoimmune disorder. Rinvoqwas the first oral treatment for Crohn’s disease to be approved. It was studied at ENCORE Borland Groover Clinical Research. Inpefa (sotagliflozin) has been approved as a daily pill to reduce cardiovascular death, hospitalization, and urgent heart failure by 33% in patients with heart failure. It was studied at the Jacksonville Center for Clinical Research. We at ENCORE Research Group are very excited about a year of amazing approvals. It validates the hard work and effort of all of our research staff and doctors, and – most importantly – it goes to show just how important our patient volunteers are! With the help of these heroes, we have new and effective options to help with six different diseases. Many thanks to everyone who has participated in any of our clinical trials, and we’ll see you next year!
References: Abbvie. (18 May, 2023). U.S. FDA Approves RINVOQ® (upadacitinib) as a Once-Daily Pill for Moderately to Severely Active Crohn’s Disease in Adults. https://news.abbvie.com/news/press-releases/us-fda-approves-rinvoq-upadacitinib-as-once-daily-pill-for-moderately-to-severely-active-crohns-disease-in-adults.htm Astellas. (13 May, 2023). Astellas’ VEOZAHTM (fezolinetant) Approved by U.S. FDA for Treatment of Vasomotor Symptoms Due to Menopause. https://www.astellas.com/en/system/files/news/2023-05/20230513_en_1.pdf GSK plc. (3 May, 2023). US FDA approves GSK’s Arexvy, the world’s first respiratory syncytial virus (RSV) vaccine for older adults. https://www.gsk.com/en-gb/media/press-releases/us-fda-approves-gsk-s-arexvy-the-world-s-first-respiratory-syncytial-virus-rsv-vaccine-for-older-adults/ Lexicon Pharmaceuticals. (26 May, 2023). Lexicon Announces FDA Approval of INPEFA™ (Sotagliflozin) for Treatment of Heart Failure. https://www.lexpharma.com/media-center/news/2023-05-26-lexicon-announces-fda-approval-of-inpefa-sotagliflozin-for-treatment-of-heart-failure Schneider, M., Narciso-Abraham, M., Hadl, S., McMahon, R., Toepfer, S., Fuchs, U., … & Wressnigg, N. (2023). Safety and immunogenicity of a single-shot live-attenuated chikungunya vaccine: a double-blind, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)00641-4/fulltext Seres Therapeutics, Nestle Health Science. (26 April, 2023). Seres Therapeutics and Nestlé Health Science Announce FDA Approval of VOWST™ (fecal microbiota spores, live-brpk) for Prevention of Recurrence of C. difficile Infection in Adults Following Antibacterial Treatment for Recurrent CDI. https://www.nestlehealthscience.us/stories/seres-therapeutics-and-nestle-health-science-announce-fda-approval-vowst Velena SE. (10 November, 2023). Valneva Announces U.S. FDA Approval of World’s First Chikungunya Vaccine, IXCHIQ®. https://valneva.com/wp-content/uploads/2023/11/2023_11_10_BLA_Approval_PR_EN_Final_.pdf U.S. Food & Drug Administration. (27 November, 2023). 2023 Biological License Application Approvals. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/2023-biological-license-application-approvals U.S. Food & Drug Administration. (19 December, 2023). Novel Drug Approvals for 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023
Scroll down to listen to this article.
Listen to the article here:
May 20th is Clinical Trials Day. Of course, we are biased and think it should become a national holiday, but until that day arrives, let’s celebrate the day by studying a clinical trial designed by our founder and CEO, Dr. Michael J. Koren. The trial we will study, published in 2006, was called the Aggressive Lipid-Lowering Initiation Abates New Cardiac Events, or ALLIANCE. In case you are unaware, the sponsors of clinical trials are all very clever and enjoy coming up with neat acronyms. ALLIANCE was a large, 16-center study conducted around the turn of the millennium and enrolled 2,442 patients. All patients had coronary heart disease – blockage of a major blood vessel to the heart. The study was designed to determine the difference in outcomes for patients with coronary heart disease when taking atorvastatin, marketed as Lipitor, at a full dose of 80 mg versus usual treatment with statins or other cholesterol drugs. Patients treated with high dose atorvastatin were monitored to keep their LDL less than 80 mg/dL. Outcomes in a clinical trial are the variables measured: what researchers are monitoring for change. Typical outcomes will include things like changes in symptoms, cure, or death. The ALLIANCE patients had coronary heart disease, so the main outcomes being monitored were: This study was significant because it looked at the difference between medications given in a clinical trial setting and “usual care” for people enrolled in managed care health plans (they all had insurance). The study results were, frankly, amazing. In the “usual care” group, patients saw their average LDL levels drop by over 23 mg/dL, a significant decrease. These are nice results, but the “more aggressively treated” group did even better. They saw LDL levels drop by more than 34 mg/dL, a 50% greater reduction! Furthermore, the aggressive treatment led to 17% fewer events, including a whopping 47% fewer heart attacks! Needless to say, the aggressive atorvastatin treatment was a resounding success. The stated goal of the study was to discover the effectiveness of the investigational medication, but the ALLIANCE study also measured the difference between clinical trials and standard care. The aggressive treatment group received clinical-trial-level care with specialist medical professionals and frequent check-ins. Patients had a lot of contact, and researchers were interested in any and all health changes. The study showed great success for the sponsor. It helped make Lipitor the best-selling medication of all time (recently broken by Humira). It gave us solid evidence that the clinical trial process doesn’t just lead to new potential medications but also potentially better health outcomes for patients. Thanks, Dr. Koren, for taking leadership of this trial and the ENCORE Research Group. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
Sources: Koren, M. J., Hunninghake, D. B., & Alliance Investigators. (2004). Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. Journal of the American College of Cardiology, 44(9), 1772-1779. https://www.sciencedirect.com/science/article/pii/S0735109704016365
Scroll down to listen to this article.
Listen to the article here:
Clinical research is vital in developing knowledge geared toward improving future health practices. It is often the only feasible solution to medical conditions where standard treatment has failed. Research can be groundbreaking and progressive but study populations lack diversity, especially regarding demographics such as race, ethnicity, age, and sex. Which begs the question: “Why is diversity important to research?” Simply put, various subsets of people can process the same medication differently. Through the clinical research process (guided by strict safety protocols), researchers gather data on how participants experience the same investigational product (IP). Suppose a clinical trial includes people from many different age, gender, and racial/ethnic groups. In that case, researchers will then have data on how several groups of people respond to the same product based on those differences. When these new products reach the market, doctors will know what works best, what doesn’t, and for whom based on those findings. Through research, doctors learned that it is best to prescribe particular medications based on patients’ ethnicity/racial background when treating hypertension. As we move forward, the clinical research community thrives on increasing the participation of all minority age, gender, and racial/ethnic groups to improve the safety profile of medical products. The US government must lead this effort through legislation. At ENCORE, we recognize that to benefit the communities we serve, we must take the steps necessary to best represent ALL in the trials we conduct. However, this requires overcoming numerous challenges, including long-standing mistrust and economic barriers which prevent minority groups from accessing and participating in research. Even though clinical trials have evolved from historically unethical practices involving minority racial groups to a transparent process where participant safety and protection are paramount, there is still a significant lack of trust on the part of potential study subjects. Especially for those who are dubious, establishing credibility and integrity within clinical research practice is crucial to growing minority group participation. We believe that physicians, especially those who serve ethnically/racially variant communities, play a significant role in achieving Diversity in clinical trials by bridging trust between researchers and minority participants. ENCORE physicians have dedicated their efforts to working alongside primary care physicians and specialists who serve these communities; to provide them with research material relevant to their practice. Doctors then have the information to make informed recommendations on whether a particular clinical trial is appropriate for their patients. Not only are people more likely to be confident in the recommendations coming from their doctors, but doctors find themselves better prepared to help patients who have failed on current therapeutic approaches. Unlike when seeking intervention via traditional means, economic hindrance isn’t a preventative factor when one chooses to participate in clinical research. Healthcare costs are a significant burden to many; however, all of the investigational medical products available here at ENCORE cost our patients nothing. All study-related materials, evaluations, blood tests, and imaging are done for free. Study participants will never be billed. Sponsors such as pharmaceutical companies, governments, and foundations fund medical research through study grants. Grants provide the funding to conduct studies at local research sites, so the cost is not transferred to the volunteers. This allows people experiencing financial constraints or without health insurance the opportunity to receive potentially groundbreaking medical treatment at no cost to them. A small monetary compensation is often provided for participants’ time and inconvenience associated with participating in a clinical study. Payment is kept within a reasonable amount to prevent enticement and undue influence on participants. Despite these steps, we recognize that much more needs to be done to garner diverse research participants in all our trials. Appropriately representing Diversity in clinical trials is an ethical and medical obligation that bounds all stakeholders. Critical players like research sponsors, investigators, referring physicians, coordinators, recruiters, and patients must work collaboratively to achieve this goal. A multi-level stakeholder approach can be more successful than one which addresses a single barrier or involves individual stakeholders. As a research facility, our responsibility in achieving increased Diversity amongst trial participants will build on our investments towards nurturing long-standing relationships. These relationships are between sponsors, community members, our diverse pool of staff and physicians, and our commitment to engagement and learning from diverse patient groups. With this approach, we are confident that trial enrollment will continue to become more diverse and result in a more accurate representation of the people that products are intended to treat. Albertha V. Lalljie, MBBS, MPH
Sticks and stones may break my bones, but words can never hurt me. But what if words could hurt you? Meet the nocebo effect, the evil twin of placebo. The nocebo effect is an increase in negative symptoms in response to patient expectations. This occurs in both clinical trials and normal clinical care. In clinical trials, this frequently presents as adverse events occurring during a placebo administration (such as a sugar pill instead of a blood pressure med), but it’s important to note that nothing needs to be administered for an increase in symptoms. Nocebo effects may sound like no big deal, but symptoms can be significant. In clinical trials, 4-26% of patients who discontinue medication do so because of nocebo reactions. Unfortunately this burden isn’t evenly spread. Women experience an outsized effect, as do those suffering from certain psychiatric illnesses, such as anxiety and depression. Furthermore, both pessimistic and type A individuals experience higher rates of nocebo effect. So how does it work, and are the effects real? Nocebo effects are due to our own expectations. When a doctor, nurse, or researcher states the potential side effects for a medication or procedure, patients are more likely to experience those effects. This has been shown in several anecdotal settings, but also in multiple research studies. During the COVID clinical trials, patients were reporting serious side effects that tracked popular media descriptions – even when they were given saline instead of the real vaccine! These effects show the power of negative expectations, one of the three psychological mechanisms underpinning the nocebo effect. Negative results are a direct result of expectations. Researchers in one study tested the effect of word phrasing on pain outcomes. When pregnant women were preparing to get an anesthesia injection the researchers talked them through what might happen. Half the patients were given the standard spiel, “You are going to feel a big sting and burn in your back now”. The other half were given the same information in much more neutral language: “We are going to inject the local anesthetic that will numb the area.“ The neutral language group experienced significantly less pain during the injection – just from phrasing! Scientists think the negative expectations might increase focus on symptoms. The expectations don’t have to come just from doctors or nurses, however. Seeing someone else suffer a side effect or hearing stories can produce the same effect! Two other psychological underpinnings for the nocebo effect are less direct. Misattribution is the blaming of normal aches and pains to a new medicine. Progressive disease effects can also be misattributed to a placebo medication. Finally, conditioning has a large effect. Conditioning is the long-term associations we make between seemingly related things. Some patients feel nauseous at the smell of a hospital, for instance. This can also be very specific; the color of a pill can induce distinct side effects. Patients taking blue sugar pills are more likely to experience and report drowsiness than those taking pink sugar pills. Psychology shows us the framework for understanding what’s happening, but what’s going on under the hoodie? Our brains experience changes at suggestions. Scientists think these changes may be due in large part to anticipatory anxiety. Anticipatory anxiety activates at least two pathways in the brain: pain and stress. Part of the pain pathway is called the CCKergic pronociceptive [pro-no-si-cep-tive] system. It is activated by a peptide called cholecystokinin [kow·luh·si·stuh·kai·nuhn] (CCK), and increases our perception of pain at the spinal level. This undermines anesthesia and increases our feelings of pain. The stress pathway moves through a few brain regions along the hypothalamus–pituitary–adrenal (HPA) axis and produces cortisol. Cortisol is a hormone that causes all of the classic signs of stress – increased heart rate and blood pressure, sweating and breathing, among others. The activation of these two major pathways primes our brain and bodies to experience worse symptoms – especially those we are expecting. So what can be done? Well, reading this article is a great start! Around 75% of patients haven’t heard of or don’t believe in the nocebo effect, even though they experience it. Thinking about risks in terms of percentages instead of raw numbers can help. Four in a thousand may increase anxiety more than 0.4%.Other methods may be in the hands of medical professionals. Positive framing, such as with the injection example above, can make a lot of difference. In addition, identifying risky patients may help with how information is presented. Fortunately, understanding the mechanistic nature behind the nocebo effect can help lessen your anxiety – and symptoms! Written By Benton Lowey-Ball, BS Behavioral Neuroscience
Sources: Kong, J., Gollub, R. L., Polich, G., Kirsch, I., LaViolette, P., Vangel, M., … & Kaptchuk, T. J. (2008). A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. Journal of Neuroscience, 28(49), 13354-13362. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2649754/ Planès, S., Villier, C., & Mallaret, M. (2016). The nocebo effect of drugs. Pharmacology research & perspectives, 4(2), e00208. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4804316/ Varelmann, D., Pancaro, C., Cappiello, E. C., & Camann, W. R. (2010). Nocebo-induced hyperalgesia during local anesthetic injection. Anesthesia & Analgesia, 110(3), 868-870. https://doi.org/10.1213/ANE.0b013e3181cc5727
Listen to the article here:
Our patients are enthusiastic and excited to take part in clinical research. There are a variety of reasons a patient would want to participate in a clinical trial: they join to benefit future generations, to advance medicine, to get medical help and compensation, and to increase diversity. The most frequent reason for joining a clinical study, however, is to help others. Clinical research is the best framework for ensuring the safety and effectiveness of new medications, devices, and procedures. This includes everyone from participants of phase 1 clinical trials to final consumers after FDA approval. But what does the process actually look like for patients? People hear about us from a wide variety of sources: advertisements, community outreach programs, the internet, and personal referrals from family and friends. Thousands of our patients are referred to us by friends, family, and their own physicians. People’s great experiences with us make them very likely to recommend us to others. Most of our patients are repeat offenders. In fact, over 99% of our patients return for another study. When you are interested in an ENCORE Research Group study, our experienced and compassionate recruiters will talk with you. These experts care about your time more than anything else. They will run through a quick checklist to see if you prequalify for a study. If you prequalify, they will schedule an evaluation. They will find a time that works best for you to come in or receive a call with a research coordinator. Here the compassionate and attentive nature of ENCORE Research Group excels. You will receive forms to fill out your medical history, medications, and contact information. During your appointment, our attentive and detail-oriented staff will review your documents. They will confirm and expand on any medical conditions that may affect your participation. This step makes sure you are always safe and gives our staff personal knowledge to help you during your study. Patient safety is always our number one priority. After an evaluation, if you choose to participate, you will start the informed consent process. Here you will review the clinical trial process and the plan for your specific study. Research coordinators will explain and review a highly detailed and regulated consent form. This document informs you about the study, potential side effects, the goals and endpoints of the study, your rights, and what to expect. This is a vital step. We will also remind you that you can end participation in the study at any time for any reason. Your voluntary participation does not oblige you to continue at any point. What happens from here depends on your specific study, but some things will remain constant. Our doctors and medical staff will talk with you. Other patients describe them as professional, friendly, and compassionate. You may receive medication, a placebo, device, or undergo a procedure. This will have been explained in detail during the informed consent process. One big difference between ENCORE Research Group and a normal doctor’s office is the comprehensiveness and amount of follow-up. Our doctors give you their full, undivided attention when you are in their office. They have plenty of time and want to know the intricacies of your medical history. For most studies, our staff will periodically check up on you after you leave. We are also keenly interested in knowing if you experience any new or changing symptoms. This will continue until your study has concluded. Then, if you wish, we will contact you if you qualify for more studies. This process results in a streamlined, professional, and personal system. You get to help medicine and society, but also experience top-quality, attentive care for a variety of conditions. Join the clinical trial process with ENCORE Research Group and see why nearly all of our patients come back! By Benton Lowey-Ball, BS Behavioral Neuroscience
Most of us have heard by now that chocolate is healthy, or that a small amount is healthy, or that you can eat an infinite amount of chocolate and it will be healthy forever. Where do these claims come from, and do they add up? There is evidence of people consuming chocolate up to 1600 years ago. It is native to the Americas and was said to be the “food of the gods” in mesoamerica. Today we think of chocolate as sweet and delicious and the perfect food, but this was not always the case. Chocolate is thought to have originally been mixed with water and drunk as a bitter, spiced beverage. During the 1500’s chocolate was brought to Europe, where it was considered as exotic as Mars. Healers claimed chocolate healed diseases of the liver and stomach, and that it could help with fever. By 1631, chocolate had changed. Adding sugar was now typical, and the prescriptions for chocolate had changed as well. Chocolate in this era was used to help gain weight (likely due to sugar), stimulate the brain (likely due to caffeine), and aid in digestion. Ironically, the same benefits chocolate seemingly presented to chronically underweight pre-industrial people has become a bit of a problem for us. By the mid to late 1800’s there were investigations into the health problems associated with chocolate’s additives – milk and sugar. They found that regularly eating fatty, sugary foods might not be healthy. By the 1900’s chocolate began to be associated with obesity, tooth decay, gum disease, etc. The “dark” chocolate age had begun. By the early 2000’s, the opinion pendulum on chocolate had begun to swing back. Individual components of chocolate, such as flavanols, methylxanthines (Methyl-zan-theens) and polyphenols were shown to be beneficial to heart function in a lab. Since then there have been claims that chocolate helps everything from cardiovascular problems to metabolic ones and even cancer. It looked like chocolate was on a holiday high in medical opinion. Unfortunately, these results may have been candy-coated. Research trials haven’t shown as much benefit as in the lab. One sweet spot picked up by newspapers was an observational meta-study which looked at over 300,000 participants. This study looked for an association between chocolate consumption and coronary artery disease (CAD). They found that people who ate chocolate more than once a week (or more than 3½ times a month) had a significantly lower incidence of CAD, heart attack, heart failure, and acute coronary syndrome. It is important to note that this was not an interventional study, and only looked at associations. Additionally, this didn’t take into account the type of chocolate eaten. Finally, this study found that some negative indicators actually rose, likely due to the extra calories from fats and sugars added to chocolates. The best way to look for health benefits or drawbacks of any medicine is to do an interventional experiment – a clinical trial. This is where you compare groups randomly assigned to take chocolate or a placebo. An examination of 15 such studies where chocolate was the medicine sadly found few benefits. These studies looked for changes in: When looking at all 15 studies, there was no significant change in any of these indicators. The only significant change across studies was a decrease in triglycerides. This can be helpful, as high triglycerides can be a risk factor for CAD, stroke, and pancreatitis. Overall, however, chocolate doesn’t appear to be the miracle drug it’s been touted as for the last millennium and a half. As we have learned countless times, using randomized clinical (interventional) trials is the best and often only way to discover if medicines have the effects people claim! Interventional trials are conducted at clinical research organizations such as ours, ENCORE Research Group. We are a premier clinical research organization that has conducted more than 2,500 clinical trials over 25 years and has worldwide recognition for providing patients access to cutting edge medical research. Written by Benton Lowey-Ball, BS Behavioral Neuroscience
Sources: Krittanawong, C., Narasimhan, B., Wang, Z., Hahn, J., Virk, H. U. H., Farrell, A. M., … & Tang, W. W. (2021). Association between chocolate consumption and risk of coronary artery disease: a systematic review and meta-analysis. European journal of preventive cardiology, 28(12), e33-e35.https://doi.org/10.1177/2047487320936787 Lippi, D. (2015). Sin and pleasure: the history of chocolate in medicine. Journal of agricultural and food chemistry, 63(45), 9936-9941. Montagna, M. T., Diella, G., Triggiano, F., Caponio, G. R., Giglio, O. D., Caggiano, G., … & Portincasa, P. (2019). Chocolate,“food of the gods”: History, science, and human health. International Journal of Environmental Research and Public Health, 16(24), 4960. https://doi.org/10.3390/ijerph16244960 Tan, T. Y. C., Lim, X. Y., Yeo, J. H. H., Lee, S. W. H., & Lai, N. M. (2021). The health effects of chocolate and cocoa: A systematic review. Nutrients, 13(9), 2909. https://doi.org/10.3390/nu13092909
Listen to the article here:
Several of my friends hate flossing their teeth. They go months without flossing, which I think is pretty gross. But then an odd thing happens. About a week before their dental appointment, these same friends will start flossing. By the time they reach their appointment, they have unusually clean gums (though dentists can see through this fairly well, I’m told). On a different tone, some family members have a condition called White Coat Syndrome. When they go to the doctor’s office, their nervousness causes a spike in blood pressure or heart rate, giving deceptively high readings. What’s going on? Can psychological effects like these be used to our advantage?
The Hawthorne Effect is a term used to describe a very beneficial effect seen in clinical trials. This is named after a productivity study in Hawthorne Works, a Western Electric factory in the 1920s and 30s. The study was attempting to discover a link between the amount of light and productivity of workers. When increasing the amount of light, productivity increased. Strangely, when lowering the amount of light, productivity also increased! Researchers attributed the increase in productivity to the workers simply being observed. In research, we tend to see increased positive results for patients simply because we are observing them in a study.
Hawthorne Works
Let’s analyze a 2014 sleep study. Researchers measured 195 patients’ amount and quality of sleep at night. 81 days later, before any medical intervention, researchers measured the patients again. They found that patients slept an average of 30 minutes longer per night and had an increased quality of sleep. This was before any medication or intervention! The change was attributed to the Hawthorne Effect. Patients at ENCORE Research Group comment on the excellent quality of care they receive during clinical trials. Instead of seeing a doctor for a few minutes once a year, patients see doctors and medical staff for much longer and are encouraged or required to call and report changes in health. Quality of care is increased and makes for a pleasant and healthful patient experience. Patients in clinical trials may also experience more observation time from medical professionals due to the attention to detail that clinical trials require for data integrity in studies. Finally, patients are found to have better adherence to medication requirements while undergoing clinical trials. The increased emphasis on accuracy and adherence results in better patient outcomes, even when they are part of a placebo or standard-of-care group. In clinical trials, we see these benefits and must account for them. Randomization of patients helps spread the effect. Everyone sees increased baseline results on average; we are interested to find out if those receiving investigational treatment do even better. Join a clinical trial today and experience the Hawthorne Effect for yourself… and floss your teeth! Written by Benton Lowey-Ball, BS Behavioral Neuroscience
Sources: Benedetti, F., Carlino, E., & Piedimonte, A. (2016). Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. Lancet Neurol, 15, 736-47. http://dx.doi.org/10.1016/S1474-4422(16)00066-1 Cizza, G., Piaggi, P., Rother, K. I., Csako, G., & Sleep Extension Study Group. (2014). Hawthorne effect with transient behavioral and biochemical changes in a randomized controlled sleep extension trial of chronically short-sleeping obese adults: implications for the design and interpretation of clinical studies. PLoS One, 9(8), e104176. https://doi.org/10.1371/journal.pone.0104176 ENCORE Research Group. (2020, October 14). Hawthorne effect.[Video]. Youtube. https://www.youtube.com/watch?v=1DH7jwqFlyw Mayo, E. (1993). The human problems of an industrial civilization. The Macmillan Company. McCarney, R., Warner, J., Iliffe, S., Van Haselen, R., Griffin, M., & Fisher, P. (2007). The Hawthorne Effect: a randomised, controlled trial. BMC medical research methodology, 7(1), 1-8. https://doi.org/10.1186/1471-2288-7-30
Listen to the article here:

Alzheimer’s Disease is devastating. An estimated 6.2 million Americans age 65 and older are living with Alzheimer’s dementia in 2021. Alzheimer’s is a brain disease that causes a slow memory decline. It can also affect your thinking, problem-solving, and reasoning skills. There are ten signs that you or a loved one may be experiencing early stages of Alzheimer’s Disease. If any of these signs persist, you should schedule an appointment with your doctor and come in for a free memory screening at Jacksonville Center for Clinical Research (JCCR). A critical factor in spotting Alzheimer’s Disease’s early stages is noticing the memory loss of recently learned information. The memory loss examples include forgetting dates and events and asking the same questions multiple times. It can be difficult for a person in the early stages of Alzheimer’s to complete daily tasks. For example, they may find it difficult to find familiar locations or do simple things such as making a grocery list or remembering the rules to a favorite game. It can sometimes become difficult for those with Alzheimer’s to work with numbers. Tasks such as paying bills may get swept under the rug or have excessive errors. They also have difficulty planning things as simple as everyday errands. Alzheimer’s Disease can cause confusion and result in anxiety or panic. Frequently, they can forget where they are or how they got there. Alzheimer’s Disease and vision issues can go hand-in-hand. For example, they may show difficulty reading, balancing, or distinguishing the depth and color of objects. It can be difficult for someone with Alzheimer’s to join a conversation. They may stumble on their words. They may have trouble remembering names and often repeat themselves. Everyone loses their keys, remote, or wallet every once in a while. However, someone suffering from the early stages of Alzheimer’s may often lose these things or put them in strange places. For example, they are putting their keys in the fridge. Someone who has Alzheimer’s Disease may have poor judgment. Examples of this can be poor hygiene, trouble dealing with money, or acting irrationally. It can become difficult for people with Alzheimer’s to work or interact socially. You may notice them pulling away from normal social activities. They may start to have trouble keeping up with their favorite activity. Suffering from Alzheimer’s Disease can be extremely frustrating. It is common to experience sudden mood changes and sometimes act irrationally. They can quickly become confused, suspicious, depressed, or even fearful. It is important to remember that we can have a natural decline in cognitive ability as we age. However, when that decline disrupts daily life, it is time to see a doctor for a memory screening. Thankfully, there have been many breakthroughs in memory research, although there is still no cure for Alzheimer’s. Clinical trial studies are the only way to continue to learn about this disease in hopes of and finding a cure. If you or a loved one is currently experiencing any of these early symptoms of Alzheimer’s Disease, we encourage you to get a memory screening. ENCORE Research Group offers free memory screenings at our Jacksonville Center for Clinical Research location and has several studies enrolling for Alzheimer’s Disease. For more information, visit encoredocs.com or call 904-730-0166.
1.Memory Loss that Disrupts Daily Life
2. Difficulty Completing Normal, Daily Tasks
3. Trouble with Planning or Problem Solving
4. Confused about the Current Time or Place
5. Trouble with Vision and Depth Perception
6. Difficulty Pronouncing Words or Writing
7. Losing Important Items Often
8. Poor Judgement
9. Becoming Socially Distant
10. Mood Swings
The Hawthorne Effect is an interesting phenomenon where people alter their behavior due to the awareness of being observed. This effect was first discovered in the 1950s outside of Chicago. The experiment was done on factory workers, and it found that workers had a positive response to the extra attention given by managers who cared about them. This same phenomenon has been noticed in clinical trials as well. “When you’re doing a clinical trial, and you’re involved with something that is being observed, your patients tend to do better regardless of how they are being medically treated.” Dr. Michael Koren, CEO of ENCORE Research Group, says. For example, if a patient is in a clinical trial for weight management, they may be more likely to lose weight if they must keep a log of everything they eat and present it to their study coordinator. So what does that mean for you? It means that you have the chance to improve your health just by participating in a clinical trial, even if you happen to be on a placebo. Studies have shown that patients in clinical studies are more adamant and knowledgeable about their health in the first place. It is safe to say your health will likely improve no matter what study you participate in! To see what studies are available right now, visit our enrolling studies page!
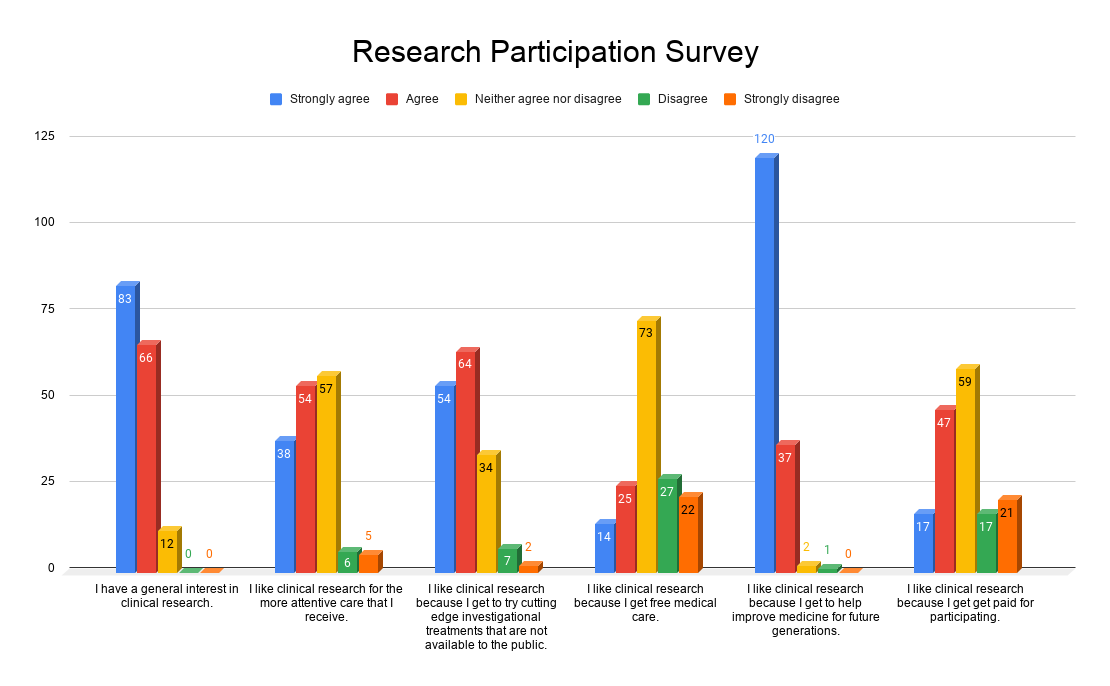
We asked, “What motivates you to participate in clinical trials?” With over 160 responses, the answer is clear. People who participate in clinical trials are dedicated to helping others by improving medicine for future generations.
We also found that very few were participating in order to receive the stipend for time and travel. This says a lot about the type of people who are willing to participate in clinical trials. They are in it for the cutting edge treatment, and the need to help others.
There is truly only one way to improve healthcare, and that is to participate in clinical trials. Thank you to everyone who responded to our survey, and everyone who participates in these trials.
On a sailing ship in 1747, twelve sailors who had begun the voyage feeling fine were overcome with fatigue. Their gums were swollen and sore, making it difficult to eat. Their teeth were falling out. Their legs were swollen and purple from bruising.
Dr. James Lind was a passenger on that ship, and he set out to find the cause. He set up what may have been the first clinical nutrition experiment. He decided on six groups of treatments, 2 sailors in each group:
- drank one quart of cider a day
- gargled with sulfuric acid
- had two spoonfuls of vinegar, 3 times a day
- drank ½ pint seawater a day
- drank barley water
- ate two oranges and 1 lemon a day
Within six days, the sailors who ate the oranges and lemon felt better and were able to work again. The other sailors in the experiment felt worse. The ill sailors were suffering from a lack of vitamin C, now known as Scurvy. They had plenty of fresh fruits and vegetables when they first set out on the voyage. But fresh foods ran out on the long voyage, and they suffered symptoms from this lack. After this finding, sailors often brought lime juice aboard ship because it could be stored longer. This is how sailors earned the nickname “limey”.
1747 was well before the requirement of informed consent of the patient, detailed eligibility criteria, protocols and regulations, which are a foundation of today’s clinical research. Nevertheless, it is an interesting example of a method of discovering the best treatment for a disabling condition.
Scientific minds are still seeking solutions for medical problems. Modern clinical research is strictly regulated for the safety and well-being of the research volunteer. Great progress has been made in medical science over the last decades. This progress could not happen without dedicated volunteers. Participation in clinical trials can be a rewarding endeavor for both investigators and volunteers alike.
Written by: Julia Baker, RN, CCRC
Resources:
https://askabiologist.asu.edu
https://www.umass.edu/nibble/infofile/limey.html
Why do our volunteers want to participate in clinical trials? Volunteers are often motivated by a combination of several reasons. Here are 6 of the top reasons to participate.
-
The potential of finding relief from their symptoms
We can’t promise relief from symptoms due to placebo and efficacy of the medication being tested. However, the Hawthorne Effect proves that patients who participate in a research trial have better outcomes than those not participating.
-
To learn more about their condition
You might argue that to learn about a condition you can just look it up on the web, and we all do that. However, often these websites can be misleading or provide the worst-case scenario results, which don’t apply to most of us. Another option is to ask your primary physician, and that is a good thing to do. Unfortunately, physicians are often rushed or running behind and questions are forgotten. Participating in a clinical trial provides you ample one on one time with a research professional and physician so that all your questions can be answered.
-
Access to new cutting-edge treatments
When participating in a clinical trial, there is access to new cutting edge treatments that are not available yet to the public. There can even be access to medications that have been newly FDA approved, but are much too expensive to afford. Study required medications are most often provided at no cost!
-
Receiving medical care at no cost
Sponsors such as pharmaceutical companies, governments and foundations fund medical research through study grants. The grants fund local research sites for conducting the study so you don’t pay a thing. In fact, we don’t even ask you for your insurance information! Can you believe most studies compensate patients for time and travel?
-
Making a difference
Clinical Trials help shape the future of medicine and healthcare. Volunteer participation helps researchers discover more about health conditions and find better ways to treat them!
-
Moral and emotional support
Having medical conditions that others don’t necessarily understand can make some people feel alienated. When involved in research, support staff understand the patient’s condition and what they may be experiencing and can provide moral and emotional support.
We will do everything we can to help find a trial that is a good fit for interested volunteers. New clinical trials are constantly enrolling so do not be discouraged if we don’t currently have the perfect trial for you. The majority of volunteers who completed a clinical trial are interested in participating in another one, so call us and find out your reason to participate!
At ENCORE Research Group it is our mission to help every patient that walks through our doors qualify for the clinical trial of their choice. Often times we get to experience the thrill of telling our patients that they successfully qualified and will soon enroll in the study. However, this is not always the case and we understand our patients’ frustration when they decide to commit to a trial only to later find out that they do not qualify. Here at ENCORE Research Group we were curious how this situation affected their thoughts about applying for future studies. This month, curiosity got the best of us and we reached out to some of our community members to find out!
Thomas recently came to Jacksonville Center for Clinical Research (JCCR) to have an evaluation for a high triglyceride and weight loss study. Fortunately for Thomas, he was not eligible for the trial because his triglyceride level was too low. Thomas stated “it’s good that I am healthy enough not to be in this research study. But they are looking at another study that I may be interested in.” As you can see, Thomas was not discouraged that he did not qualify, but optimistic that he may qualify for a different study.
We also reached out to Latasha, who is new to research, and may qualify for a study that has a waiting list for interested participants. When discussing with her how some patients are not eligible to join a trial, she stated “I would want to know why I did not qualify, but that would not prevent me from trying to get in another trial.” Fortunately for Latasha and all of our participants, ENCORE Research Group is very transparent about the screening process and explains exactly why they may have been ineligible. Hopefully she will receive a spot in the study she applied for!
Mark was passionate about participating in a Sjogren’s research trial but did not qualify. He said “it’s not unusual for someone with Sjogren’s Disease not to qualify for studies with systemic therapies if they do not have the antibodies. But when you’re sick, your driving force is to get better for yourself and your family. I tried the conventional way but it did not work for me.” After doing more research on the specific clinical trial, Mark decided to pursue the FDA Expanded Access program. “It’s a relatively new program to help people get access to new medications.” Fortunately for Mark, he had the resources and insight to look into alternative treatment options.
We also asked an experienced researcher how she saw things. Linda Gray, site manager of the Nature Coast Clinical Research site in Inverness, Florida, has many gastrointestinal (GI) studies currently enrolling. Linda acknowledges that “some of our patients are not eligible for a study because they have mild disease, and the sponsors are looking for moderate to severe disease. If the disease is not measurable enough for objective data, we will not be able to tell if we’ve reversed it or slowed the progression. Our NASH studies include a liver biopsy to determine the extent of the disease to see if the patient is eligible.” It is unfortunate that this can limit access to drugs for those in need, but we have to believe that obtaining clear and objective data will help a greater number of people in the future.
The reality is, every clinical trial is different and has unique qualifying criteria. The pharmaceutical companies that sponsor clinical trials create the criteria in order to make the strongest case possible to the FDA on the drug’s safety and efficacy. While we would love to involve every one of our community members that are interested, it is just not always possible. The good news is that all seven of our research sites are always getting new clinical trials to enroll in! So, just because you didn’t qualify the first time doesn’t mean you won’t qualify for the next one! We look forward to working with you in the ENCORE community.
The practice of medicine has changed in major ways in recent years. Though many of these changes reflect good intentions, the real world consequences to patients often don’t match expectations. To understand this divide between reasonable intentions and the less salubrious reality from which we may collectively suffer, I’d like to share a recent anecdote that occurred at the airport when regulations ran amok.
During recent travel, I witnessed an unfortunate incident that you may have seen before. An airline gate agent stopped a member of our party and reprimanded her for carrying a small overnight suitcase, a computer bag and her pocketbook. The gate agent, working on behalf of the airline, stated that she had to follow FAA (Federal Aviation Authority) rules and only allow two bags on board even though the computer bag and pocket book were small. Unfortunately, when asked to “consolidate” the three bags into two, we had a problem. No bag really fit into any of the other bags. The agent wouldn’t “gate check” any of the bags so that we could pick it up when exiting the plane and running off to our next flight. Further, our discussion with the gate agent failed to alleviate our concern that, if checked to its “final destination,” we wouldn’t see a checked piece of luggage any time soon given the shortness of time for our connection.
The solution that followed satisfied no one. Over the next 10 minutes, embarking passengers stepped around the contents of the three bags which littered the entrance to the Jetway. At first, the gate agents ignored the situation, but as tempers flared due to the obstruction of foot traffic, an agent “helped” by aggressively stuffing the computer bag into the overnight bag. A busted zipper later, the bulging overnight bag limped down the Jetway led by a very unhappy customer.
Does this scenario prove airline excellence because the agents showed how well they can comply with government rules? Hardly! Most folks would conclude that this messy scenario wasn’t necessary. In complex situations, the empowerment of professionals to act judiciously given a set of circumstances leads to excellence. The above scenario required the gate agents to apply context while making an overall effort to comply with government regulations. Unfortunately an excellent result didn’t happen in this case.
Similarly, we face the issue of context and judicious interpretation every day in medicine. As a common example, computerized medical records, a well-intended effort to characterize complex information, often fail to convey the true story of a patient or the nuances that make each of us unique. Summarized, bullet point information can easily miss the point. The great composer Mozart famously observed that musical excellence doesn’t lie in the notes but actually in the space and timing in between the sounds. Medical excellence involves a similar concept. Health care providers must read “in between the lines” and understand and respond both to what is and isn’t stated.
What this concept means to the average patient depends on the circumstances. For example, some people with neck pain may need consideration for a heart condition (angina) whereas others should check in immediately for an MRI of the spine and others should book a massage. Distinguishing the underlying cause of the neck pain relies on both a description of the nature of the symptom and on understanding the quiescent pauses of relief between episodes of pain. Excellent clinicians make this distinction by asking the right questions. Excellence during the medical evaluations of headaches, arthritis, and memory problems, among other things, also require this same commitment to careful questioning.
Clinical research promotes excellence by demanding great attention to detail. During research programs, physician investigators and their staff members must extensively analyze many aspects of our patients’ health. This thorough analysis usually exceeds that which occurs at the time of general physician visits, a setting during which time pressed clinicians must limit their focus and move on to the next patient. Research also requires the deployment of state of the art technology. The combination of technology and attention to detail of symptoms, signs and lab values leads to an experience which most patients highly value and describe as a demonstration of medical excellence.
In sum, medical excellence involves more than compliance or automatically matching a disease with a drug. Medical excellence is a philosophy of understanding the needs of a patient and putting those needs in context through the development of an individual treatment plan. Clinical research promotes medical excellence by demanding a culture of detail and caring.