GRID VIEW
I’ve been watching horror films lately, so before we get into the main topic of lipoproteins let’s talk about blood. Blood is watery and kind of gross but very useful. I like to think of the bloodstream as a highway we use to transport nutrients, cells, molecules, water, and waste to and from all the cells in the body. One problem with blood is that it can’t transport things that don’t dissolve in it very well. Fats, also called lipids, don’t dissolve in water, a lesson I think we all learned with the old oil and water demonstration from elementary school. Because of this, the body bundles lipids into little packages that can dissolve in watery blood. These packages combine water-repelling lipids with special proteins that organize them so they can flow through the blood. These lipid-protein packages are called lipoproteins. They have more functions than just transport, but that’s the one we’re focusing on today. Lipoproteins aren’t just determined by their size, however. Connected to the lipoproteins are other special proteins called apolipoproteins (apo– meaning “next to” or “away”). These determine how lipoproteins form, act, are recognized, and are broken down. One dangerous lipoprotein variant is apolipoprotein (a), usually shortened to apo(a). When this attaches to an LDL-like particle, it is called Lp(a). Since the names are important and the letter “a” is common in this space, it is usually pronounced “Lp little a.” Lp(a) is bad news. When apo(a) attaches to an LDL, everything changes. The density of Lp(a) changes and it is more likely to clog the bloodstream. High amounts of low-density lipoproteins (including Lp(a)) can cause a lot of damage to the cardiovascular system, increasing the chances of serious cardiovascular events like cardiovascular disease, heart attack, and stroke. Even worse, the body can’t break down Lp(a) the same way it does LDL, so the problems of high “bad” cholesterol are compounded. Because of this, classic methods of controlling cholesterol – lifestyle changes like diet and exercise, statins, and other medication typically have no effect on Lp(a) levels! High Lp(a) is a serious health problem, affecting around 20% of people. Levels of Lp(a) in the bloodstream can vary up to 1000x from person to person! The highest levels are seen in Black and South Asian populations. As stated earlier, Lp(a) levels are unaffected by normal risk factors. Instead, Lp(a) is genetically controlled. If your parents have elevated Lp(a), it is likely you will too. The problem is one of several genetic mutations that affect the amount of Lp(a) created. As we discovered earlier, Lp(a) doesn’t break down like normal LDL, so levels are primarily determined by how much is produced. So what can we do if we have high Lp(a)? As unintuitive as it sounds, diet and exercise are still good options! This isn’t because they affect Lp(a) levels, but because a healthy lifestyle can help protect your heart. Medications that protect the cardiovascular system may also be protective against high Lp(a) levels. Currently, in extreme cases, some patients may undergo a process called lipoprotein apheresis. This is where the blood is removed from the body and lipoproteins are physically separated from the blood before it is returned to the body, just like a good horror movie! Clinical trials are investigating methods of enhancing the body’s ability to break down Lp(a) or disrupt the production of Lp(a). Production may be targeted by disrupting the body’s ability to produce apo(a)! Consider joining a research study to help find new treatment options for high Lp(a). Also, with Lp(a) day approaching on March 24th, impress all your friends with your new pedantic vocabulary. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: Biggerstaff, K. D., & Wooten, J. S. (2004). Understanding lipoproteins as transporters of cholesterol and other lipids. Advances in physiology education, 28(3), 105-106. https://journals.physiology.org/doi/full/10.1152/advan.00048.2003 Devaraj, S., Semaan, J. R., & Jialal, I. (2019). Biochemistry, apolipoprotein B. https://europepmc.org/article/NBK/nbk538139 Feingold, K. R. (2024). Introduction to lipids and lipoproteins. Endotext [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK305896/ Kamstrup, P. R., Neely, R. D. G., Nissen, S., Landmesser, U., Haghikia, A., Costa-Scharplatz, M., … & Nordestgaard, B. G. (2024). Lipoprotein (a) and cardiovascular disease: sifting the evidence to guide future research. European Journal of Preventive Cardiology, zwae032. https://academic.oup.com/eurjpc/advance-article/doi/10.1093/eurjpc/zwae032/7585314 Koschinsky, M. L., Stroes, E. S., & Kronenberg, F. (2023). Daring to dream: Targeting lipoprotein (a) as a causal and risk-enhancing factor. Pharmacological Research, 106843. https://www.sciencedirect.com/science/article/pii/S1043661823001998 Lampsas, S., Xenou, M., Oikonomou, E., Pantelidis, P., Lysandrou, A., Sarantos, S., … & Siasos, G. (2023). Lipoprotein (a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules, 28(3), 969. https://www.mdpi.com/1420-3049/28/3/969 Schmidt, K., Noureen, A., Kronenberg, F., & Utermann, G. (2016). Structure, function, and genetics of lipoprotein (a). Journal of lipid research, 57(8), 1339-1359. https://www.jlr.org/article/S0022-2275(20)35208-1/fulltext
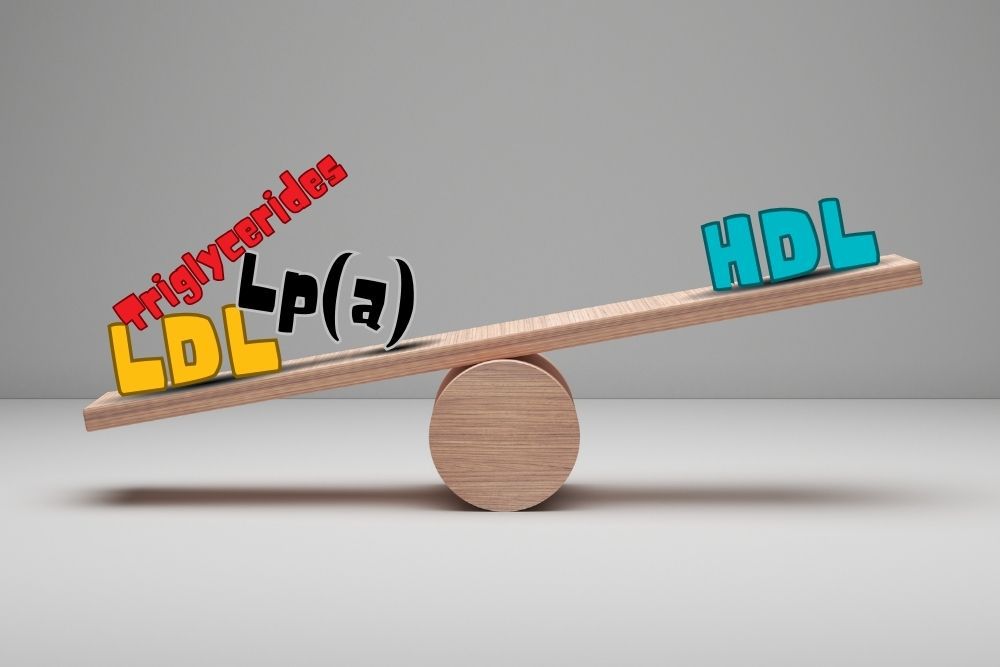
Lipoproteins are very neat. The outside is a membrane very similar to a human cell. The inside is a mix of lipids, including triglycerides and cholesterol, and the proteins hold everything together. Lipoproteins travel through the bloodstream, carrying important lipids to cells all over the body. They can be sorted by size and “density,” but density is defined differently here, more like buoyancy. Imagine a pot of water. Olive oil is almost entirely made of lipids, and if you pour it into the pot of water it floats on the surface; it’s low-density. A steak contains a lot of fatty lipids, but there’s also a ton of protein in steak. Toss it in a pot of water and it sinks (and becomes gross); it’s high-density. Lipoprotein density is more about the ratio of lipid to protein. Even more fascinating is that the ratio of lipid to protein can change over time! A very low-density lipoprotein (VLDL) will deliver triglycerides (a type of lipid) from the liver to cells, lose some density, and may become an intermediate-density lipoprotein (IDL). These are proportionally higher in cholesterol. An IDL may deliver more lipids to become a low-density lipoprotein (LDL). LDL is the primary transporter of cholesterol through the body and delivers it to cells, which use cholesterol for some essential purposes, including maintaining the membrane that surrounds cells. High-density lipoproteins (HDL) work in reverse. They transport cholesterol from the cells back to the liver, getting larger (and lower in density) as they pick up more material.Listen to the article here:
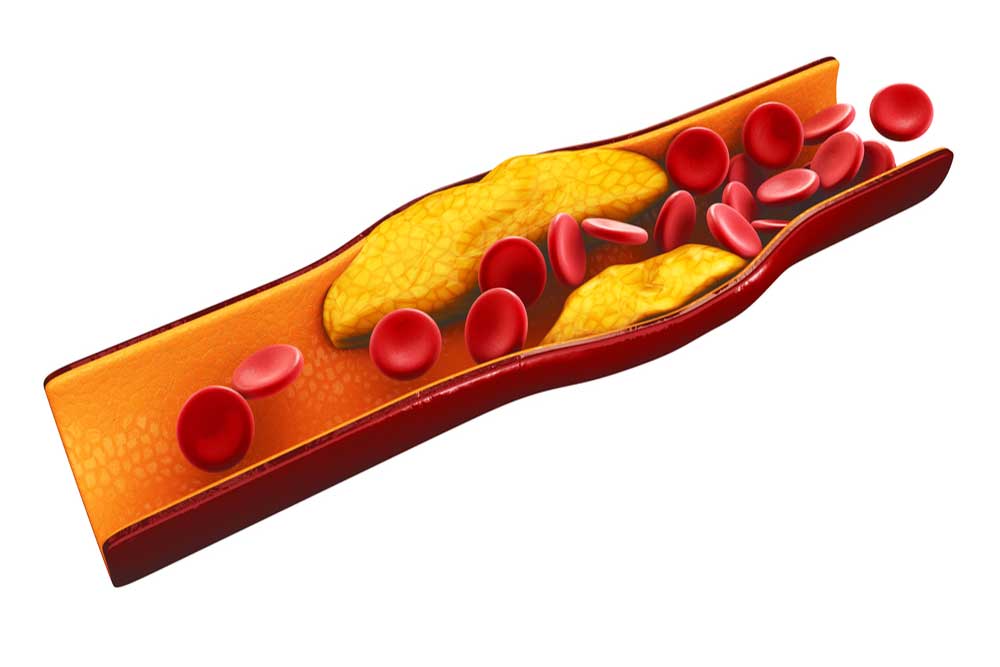
Fats are necessary for survival. In the body, we call them lipids. They are needed to build the borders of our cells, create molecules and hormones, coat important neurons in the brain, protect and insulate our organs, store energy between meals, and perform many other vital functions. Lipids are fats, which don’t play well with water; think Spongebob and Squidward (or oil and water). Lipids need to be transported through the bloodstream by proteins. Lipids attach to these proteins to make lipoproteins (lipid + protein). Triglycerides are the most common type of lipid found in the body. They are used as energy for muscles and are stored in fat cells. They are transported in very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), and gut-produced chylomicrons. Cholesterols are needed for parts of our cells and are some of the building blocks of hormones, bile acids, and enzymes. Important types of cholesterol lipoproteins include low-density lipoproteins (LDL), high-density lipoproteins (HDL), and Lipoprotein(a) (Lp(a)). Our body stays healthy in part by maintaining a healthy balance of these lipids and lipoproteins. Keeping the balance of lipids is critical to our health. When it is out of whack, we experience dyslipidemia. Dys– meaning “bad,” lipid- indicating the lipids, or fats, and -emia meaning “presence in blood”. Dyslipidemia, or bad lipid presence in blood, is when the lipids in the blood are out of balance. This condition is unsettlingly common — one of every three American adults 20 years or older has dyslipidemia. Any imbalance falls under this description, but the most common and dangerous types in the USA are high LDL cholesterol, low HDL cholesterol, and high triglycerides. The prefix hyper- means “high,” so high cholesterol is called hypercholesterolemia (high cholesterol presence in blood); high triglycerides are hypertriglyceridemia (high triglyceride presence in blood); and a combination of both is simply called combined dyslipidemia. Some people suffer from hypolipidemia (low lipid presence in the blood), but it is much rarer in the USA. Because dyslipidemia is defined and diagnosed with a blood test, the underlying causes can vary, unlike conditions such as sickle cell anemia or chicken pox. Primary causes are genetic, where you inherit a risk factor. This might look like elevated Lp(a) levels, which are heavily influenced by genetics. Secondary causes are anything that may alter lipid levels, including diabetes, obesity, an unhealthy diet high in triglycerides, and lack of exercise. Regardless of the cause, the effects can be deadly. The biggest, most obvious problem is atherosclerotic cardiovascular disease (ASCVD), when cholesterols, fats, and other materials build up on the inside of our blood vessels. These buildups, called plaques, can block blood flow to the heart, brain, or other parts of the body, potentially causing heart attack, stroke, and/or pain in the body and limbs. With these outcomes in mind, solutions are critical. As with almost every non-infectious disease, some of the best methods for preventing complications are a healthy lifestyle. A diet high in vegetables, fruits, and whole grains may help. Keeping calorie counts low and moderate-to-vigorous exercise is also recommended, if possible. Beyond lifestyle, medications have helped many people. Statins like Lipitor are typically the first line of defense. They are the most widely prescribed class of drugs in the world, though not everyone can tolerate them. They inhibit an enzyme called HMG-CoA reductase. Statins can lower LDL levels by slowing the cholesterol-making process. PCSK9 is another important enzyme in the creation of cholesterol. PCSK9 inhibitors target this enzyme to help reduce cholesterol if statins aren’t working. Bempedoic acid and icosapent ethyl may also help reduce the amount of cholesterol the liver makes. Ezetimibe (uh·zeh·tuh·mibe) reduces the amount of cholesterol absorbed. Beyond lowering lipid levels, other medications may help reduce the risk of cardiovascular disease. Finally, if medications aren’t tolerated or effective enough, blood plasma can be removed, cleaned outside the body, and pumped back in using a process called plasmapheresis. Fats may be necessary for survival, but too many in the bloodstream can be deadly. Staff Writer / Editor Benton Lowey-Ball, BS, BFA
References: Berberich, A. J., & Hegele, R. A. (2022). A modern approach to dyslipidemia. Endocrine Reviews, 43(4), 611-653. https://academic.oup.com/edrv/article/43/4/611/6408399?login=true Feingold, K. R. (2015). Introduction to lipids and lipoproteins. https://www.ncbi.nlm.nih.gov/books/NBK305896/ Pappan, N., & Rehman, A. (2023). Dyslipidemia. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560891/ Pokhrel, B., Yuet, W. C., & Levine, S. N. (2017). PCSK9 inhibitors.https://www.ncbi.nlm.nih.gov/books/NBK448100/
Scroll down to listen to this article.
Listen to the article here:
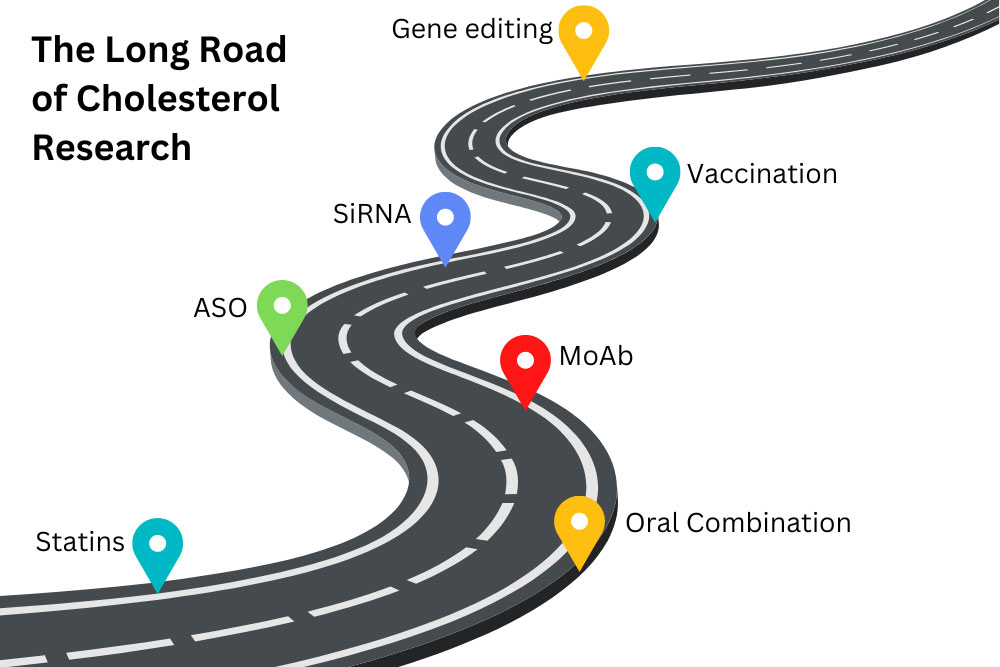
Cardiovascular disease has remained the number one cause of death worldwide. Multiple clinical trials have revealed that a common and modifiable risk factor for cardiovascular disease is high cholesterol, and if a person lowers their cholesterol, they can lower their risk for heart-related diseases. Most of us have heard of cholesterol, but what is it? Why is having too much cholesterol a bad thing? How do we get cholesterol in our bodies? What can you do to lower your cholesterol to healthy levels? Cholesterols are a broad and useful type of fat found in the body. The body needs them to create hormones, essential vitamins (like vitamin D), and other molecules. They float on the surface of our cells, helping to maintain the structure and function of cell barriers. Cholesterols regulate cell activity and act outside of cells. They insulate the neurons in our brain, allowing us to think. In fact, cholesterol is so important to daily function, that every cell in the body can make cholesterol from basic materials, except your eyelashes! There are times when cholesterol is downright bad. LDL cholesterol and Lipoprotein a [Lp(a)] have some particularly sticky portions that can get stuck to the inside of our bloodstream. We call one of these portions ApoB. Sticky cholesterol obstructs blood flow in the form of plaques. Without help, this leads to atherosclerosis, scarring, and hardening of the arteries. Atherosclerosis further cascades into cardiovascular disease, clots, heart attacks, and stroke. This is very bad. Unfortunately, it is also very common; atherosclerosis in the neck is found in ¼ of people worldwide. Lowering excess cholesterol is a global health concern. Our liver creates enough cholesterol to supply our bodies. We are also able to absorb cholesterol from our diets and make some in other cells. The most effective methods of reducing cholesterol are lifestyle and diet changes. However, for some people, diet and exercise don’t seem to budge their cholesterol numbers at all. For others, the ability to exercise and dietary restrictions may be limited. This is where medications can step in. To understand how a medication may reduce LDL and/or Lp(a), we need to learn a bit about how the body makes things from DNA. Genes are bits of DNA that contain the blueprint for a protein. Genes provide the blueprint to messenger RNA (mRNA). The mRNA translates genetic code into proteins. The cells then fold proteins into complicated, machine-like shapes. Proteins interact with molecules and other proteins to create all sorts of things for the body – including cholesterol. Clinical research has been expanding which of these steps we can target for medications. Statins are the first line treatment for reducing cholesterol. They target hydroxymethylglutaryl coenzyme A (HMG-CoA). HMG-CoA is a protein used to construct cholesterol molecules. Reducing HMG-CoA slows the body’s ability to create cholesterol, lowering cholesterol levels. Statins block the production of the “bad” LDL-C cholesterol and lower levels by as much as 60%. The benefits for statins to reduce cardiovascular events have been proven in multiple clinical trials over a diverse patient population. Other oral medications, including ezetimibe and bempedoic acid, can be taken with statins. Ezetimibe can lower LDL-C levels by approximately 20% by inhibiting cholesterol absorption in the intestines, making it a useful add-on medication when statins alone are insufficient. Bempedoic acid can lower LDL-C by 15-25% by decreasing cholesterol synthesis in the liver. Because bempedoic acid is converted to an enzyme found only in the liver and not the muscles (like statins), it is often an alternative for patients who have statin-associated muscle myalgias. Monoclonal antibodies (MoAbs) are a newer class of medication. MoAbs like alirocumab and evolocumab act like signaling molecules. These two stay outside of cells and tell the liver to produce less of the protein PCSK9. Controlling PCSK9 is a newer method of changing a person’s cholesterol profile. PCSK9 controls how much extra LDL cholesterol is absorbed and recycled by cells. MoAb medications affect this by targeting signaling receptors on the outside of the liver. Even newer medications target the process by which genes get turned on inside the cells. They are called gene silencing therapies because they aim to “silence” the gene’s effects. Antisense oligonucleotides (ASOs) and small interfering RNA (siRNA) stop the liver from producing functional LDL or Lp(a) mRNA molecules. These act at different, very early stages of the cholesterol process. In addition, specialized packaging on the medications deliver them to the liver and not other cells. This can make for very targeted medications that (hopefully) have fewer side effects. Inclisiran is the first FDA-approved siRNA therapy to lower LDL cholesterol. It is a subcutaneous injection taken twice a year. Imagine going to your physician’s office just twice a year to get your “cholesterol vaccine”! Even more amazing, gene editing tools such as CRISPR could reduce overexpression of PCSK9 or other genes on a long-term basis. These are still in early phase trials, but the future is looking bright. Lipoprotein a,or “L-P-little-a”, or Lp(a), is a new target for decreasing the risk of cardiovascular disease. Lp(a) is genetically inherited and increases the risk for both heart disease and stroke because it can promote plaque buildup, blood clots, and inflammation. New gene silencing therapies are in clinical trials right now using both ASO and siRNA technology. Diet, lifestyle changes, and statins remain the front-line defense against high cholesterol. New medicines may work with or replace these classical defenses. As technologies move through the clinical research apparatus, we may be able to tailor custom combinations of medications for individual patients. ENCORE Research Group has been involved in every step along this path, helping to study medications in every category. Join our team and help pave the way for new medications to help combat high cholesterol!
Sources: Craig, M., Yarrarapu, S. N. S., & Dimri, M. (2018). Biochemistry, cholesterol. https://www.ncbi.nlm.nih.gov/books/NBK513326/ Fernandez-Prado, R., Perez-Gomez, M. V., & Ortiz, A. (2020). Pelacarsen for lowering lipoprotein (a): implications for patients with chronic kidney disease. Clinical Kidney Journal, 13(5), 753-757. https://doi.org/10.1093%2Fckj%2Fsfaa001 Prati, P., Vanuzzo, D., Casaroli, M., Di Chiara, A., De Biasi, F., Feruglio, G. A., & Touboul, P. J. (1992). Prevalence and determinants of carotid atherosclerosis in a general population. Stroke, 23(12), 1705-1711. https://doi.org/10.1161/01.str.23.12.1705 Tokgözoğlu, L., & Libby, P. (2022). The dawn of a new era of targeted lipid-lowering therapies. European Heart Journal. https://doi.org/10.1093/eurheartj/ehab841
Listen to the article here:
After we eat a meal, all that energy has to go somewhere. Body cells can use freely floating glucose sugar in the bloodstream, but fats are a bit trickier. Just like oil and water don’t mix, fats have trouble moving through the blood in our veins and arteries. They must be packaged inside special containers called lipoproteins in order to travel where they need to go. For fats that we eat, the fats (called triglycerides) are packaged into ultra-low-density chylomicrons by the digestive system. Our liver also processes and repackages fats. The liver makes very low-density lipoproteins (VLDL) out of triglycerides and ejects them into the bloodstream. VLDLs can then use the bloodstream to travel to fat cells or be converted into other forms of energy storage. The number of triglycerides in the bloodstream at once needs to be well regulated. For adults, fasting triglyceride levels should be under 150 mg/dL. This number decreases to below 90 mg/dL for people under 19 years of age. Unfortunately, one in ten adults have high levels, called hypertriglyceridemia. When there are too many triglycerides, they can stick to the inside of the bloodstream. They can create and contribute to hard plaques, a condition called atherosclerosis. These put stress on the cardiovascular system and can lead to atherosclerotic cardiovascular disease (ASCVD). Very high triglycerides above 500 mg/dL is called severe hypertriglyceridemia. This can lead to even more problems, including chylomicronemia, pancreatitis, and death. What contributes to high triglyceride levels? A lot, actually! A diet that is high in sugars and fats, excessive alcohol consumption, being overweight, and a sedentary lifestyle can contribute. Some conditions, such as diabetes, kidney and liver disease, and thyroid problems increase your chances. Anything that affects liver function is likely to change how the body processes fats and may increase triglycerides. This means some life-saving medications, including several cancer, hypertension, and HIV treatments may increase triglycerides. Some people have high or very high triglycerides – usually in the form of chylomicrons – even without these risk factors. This may be because of our genes. One of the major genetic culprits for increased triglycerides is a gene called APOC-3. This gene codes for a protein of the same name: Apolipoprotein C-III (apoC-III). You can tell these apart because the gene is uppercase, italicized, and uses a (3), while the protein is mostly lowercase and uses roman numerals (III). The protein apoC-III can lead to some detrimental effects. Normal triglycerides bind to a different protein, apoC-II. This helps them get broken down in the bloodstream. ApoC-III binds to triglycerides in the same place as apoC-II but makes them less able to be processed. These triglycerides build up in the bloodstream and can cause atherosclerosis and ASCVD. Scientists also have evidence that apoC-III makes triglyceride-rich molecules stickier to the arteries. ApoC-III binds to chylomicrons very well, making these fats especially resistant to breaking down. So why do we have apoC-III anyway? It turns out, not all of us do! Different people have different variations of the APOC-3 gene. Some people have a gene that produces excessive apoC-III protein, and a few have genes that produce none! People with defective APOC-3 genes seem to be just as healthy as everyone else. Maybe healthier, as their levels of triglycerides are very low, even after a fatty meal! Researchers consider a defective APOC-3 gene to be cardioprotective, meaning that it lowers the chances of heart disease. Are there methods for us to lower the production of apoC-III and our triglyceride-rich chylomicrons? It looks possible. The liver produces more apoC-III in response to high levels of blood sugar and most fats, so lowering these may help. It decreases production of apoC-III when it encounters high levels of insulin or polyunsaturated fats (such as Omega-3 fatty acids). This may be helpful, but is bad news for those with type 2 diabetes. In these patients the bloodstream has extra glucose and lacks insulin. Treating high triglycerides can be complicated. A diet low in alcohol, carbs, and fats but high in omega-3 fatty acids can help. Exercise and weight loss are often helpful. Doctors may also prescribe fibrates, nicotinic acid (niacin), or statins. Unfortunately, these medications may not work if you have excessive levels of apoC-III and high chylomicrons. A diet that is very low in fats – under 20 grams a day – has been the only option for some patients. New classes of medication may be helpful as well. Antisense oligonucleotides, gene therapy, and custom antibodies can be used to target the production of specific proteins. Antisense oligonucleotides, for instance, bind to APOC-3 mRNA in the cell, preventing it from creating apoC-III proteins. They do this with extreme specificity, targeting only the gene in question. They can also do this only in liver cells by being packaged in a special way. Drugs that target apoC-III production may be able to bring down otherwise stubbornly high triglycerides without too many side effects. A side effect of reading the ENCORE Research Group website is learning about these new medicines and when they may be available for you in a trial!
Sources: Alves-Bezerra, M., & Cohen, D. E. (2017). Triglyceride metabolism in the liver. Comprehensive Physiology, 8(1), 1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6376873/ Goldberg, R. B., & Chait, A. (2020). A comprehensive update on the chylomicronemia syndrome. Frontiers in endocrinology, 11, 593931. https://doi.org/10.3389/fendo.2020.593931 National Institute of Health, National Heart, Lung, and Blood Institute. (April 7, 2022). High blood triglycerides. U.S. Department of Health and Human Services. https://www.nhlbi.nih.gov/health/high-blood-triglycerides Rahmany, S., & Jialal, I. (July 18, 2022). Biochemistry, Chylomicron. https://www.ncbi.nlm.nih.gov/books/NBK545157/ Taskinen, M. R., Packard, C. J., & Borén, J. (2019). Emerging evidence that ApoC-III inhibitors provide novel options to reduce the residual CVD. Current atherosclerosis reports, 21(8), 1-10. https://doi.org/10.1007/s11883-019-0791-9
Listen to the article here:
The Role of Apolipoprotein C-III (apoC-III) in Atherosclerosis and Cardiovascular Disease
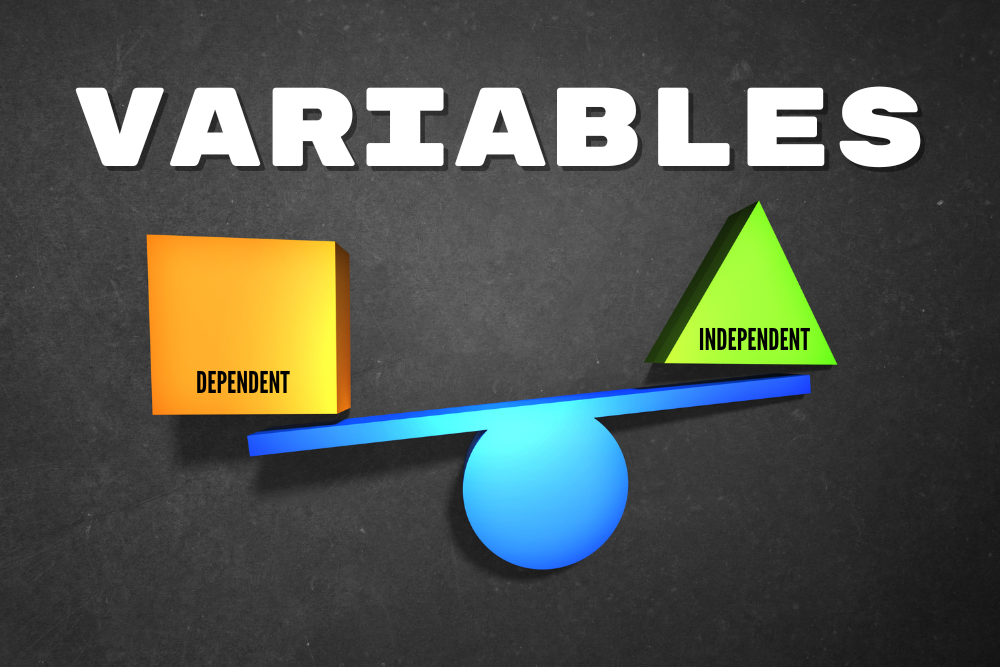
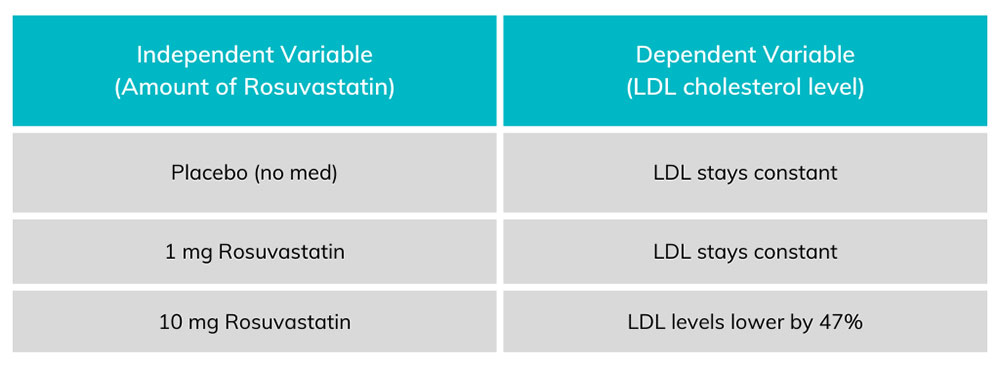
In science and medicine we measure if and how well things work using measurements. This idea may sound simple, but it’s often a challenge to find out exactly what to measure – and how. We typically measure things that can change – things that can vary. We call these things variables. Variables can be broadly split into two major categories: dependent and independent. Either type of variable can change, the difference is what changes them. Independent variables are changed by researchers, particularly in clinical (patient) research. This variable in a medical research study is what we are testing. The changes to an independent variable may include dose, length, and method of drug delivery. We evaluate independent variables that may change outcomes of the people in a study – but sometimes they do not. In order to understand the effect of medicines, researchers test the medicine against a control. The control could be a placebo (something that has no effect) or a standard of care (the current normal medicine). Dependent variables are what we expect to change during a trial. In a clinical research, we may expect changes in blood pressure, cholesterol levels, disease symptoms, mortality, and other categories. In a well designed study, we assess changes in the dependent variables related to changes in the independent variables. There is always the chance that the dependent variables are changed by other things, however. A patient might take a new blood pressure medicine but retire from their job. The reduced stress could decrease their blood pressure even if the medicine did not. Because of individual changes in people’s circumstances, researchers use statistics to find trends. If your blood pressure medicine was only studied on the one person above, you might have erroneous results. Instead clinical trials have dozens, hundreds, or even thousands of participants. With large populations these little differences get figured out. One person might retire, but another might get fired, having an opposite effect. Altogether, statistical analysis can help discover if any changes in the dependent variable are due to the effects of the independent ones.
Chart 1. Each amount of Rosuvastatin on the left corresponds to an amount of LDL on the right. The dependent variable (LDL levels) change in proportion to the amount of independent variable (rosuvastatin) taken by the patient.
Other variables exist in a study. The most concerning of these variables is known as a confounding variable. This is a variable that can undermine the study at a fundamental level. A confounding variable can be introduced by researchers and might include things like placing all overweight patients in the 10 mg group and all underweight people in the placebo group. ENCORE Research Group (and any legitimate clinical research group) avoids confounding variables and bias by randomizing patients. Patients are randomized through an impartial method (usually a computer program) which will randomly place patients into any of the test groups. By randomizing patients, we can avoid the most concerning confounding variables and make sure we are studying what we intend to! To learn more about the clinical trial process, call our Recruitment Team at (904) 730-0166. Written by Benton Lowey-Ball, BS Behavioral Neuroscience
Sources: Schweiger, C. (2003). Clinical trials with rosuvastatin: efficacy and safety of its use. Italian Heart Journal: Official Journal of the Italian Federation of Cardiology, 4, 33S-46S. https://pubmed.ncbi.nlm.nih.gov/14983745/ Stewart, P. A. (1978). Independent and dependent variables of acid-base control. Respiration physiology, 33(1), 9-26. https://www.nlm.nih.gov/nichsr/stats_tutorial/section2/mod4_variables.html
Listen to the article here:
If someone in your family had a heart attack or stroke before the age of 60, you could be at risk and might want to have your blood tested for this little-known hereditary risk factor, Lp(a). Cardiovascular disease remains the leading cause of death in the United States, even during the COVID-19 pandemic. Determining and reducing the risk factors for cardiovascular disease is critical. Lipoprotein(a), also called Lp(a), pronounced “LP Little a” is a particularly dangerous culprit. Its levels are controlled by a single gene, and a single genetic variation in this gene is enough to drastically change Lp(a) levels. Unfortunately, since it is genetically determined, diet, exercise, and lifestyle have little or no effect on Lp(a) levels. High Lp(a) can contribute to several cardiovascular conditions. These include a two to three times increase in the risk of developing: Lp(a) has been referred to as the evil twin of the more familiar LDL (bad) cholesterol and is a triple threat because it is: There are two methods of measuring Lp(a). The most common method of measuring Lp(a) is by mass, in mg/dL. Measuring how many individual particles, regardless of size, is another method and is measured in nmol/L. It is important to know which method was used when understanding your numbers. If you have never had your Lp(a) level checked, we offer Lp(a) testing to our ENCORE community for those who pre-qualify (call for details). Currently, there are no approved therapies to lower Lp(a) levels and reduce one’s risk. However, three exciting therapies are currently being studied in clinical trials at ENCORE Research Group sites across Florida. The good news is that because of clinical research and your involvement, we have new treatments for elevated Lp(a) on the horizon! Written by Benton Lowey-Ball, BS Behavioral Neuroscience
Sources: Kamstrup, P. R. (2021). Lipoprotein (a) and cardiovascular disease. Clinical chemistry, 67(1), 154-166. https://doi.org/10.1093/clinchem/hvaa247 Miksenas, H., Januzzi, J. L., & Natarajan, P. (2021). Lipoprotein (a) and cardiovascular diseases. JAMA, 326(4), 352-353. doi:10.1001/jama.2021.3632 Health.harvard.edu Amgenscience.com
Listen to the article here:
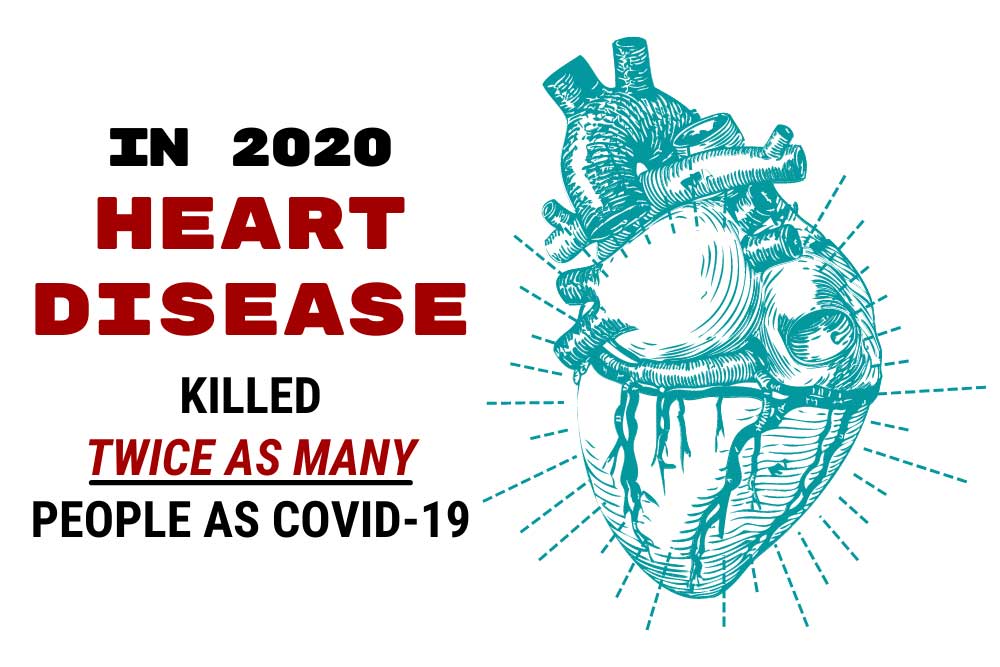
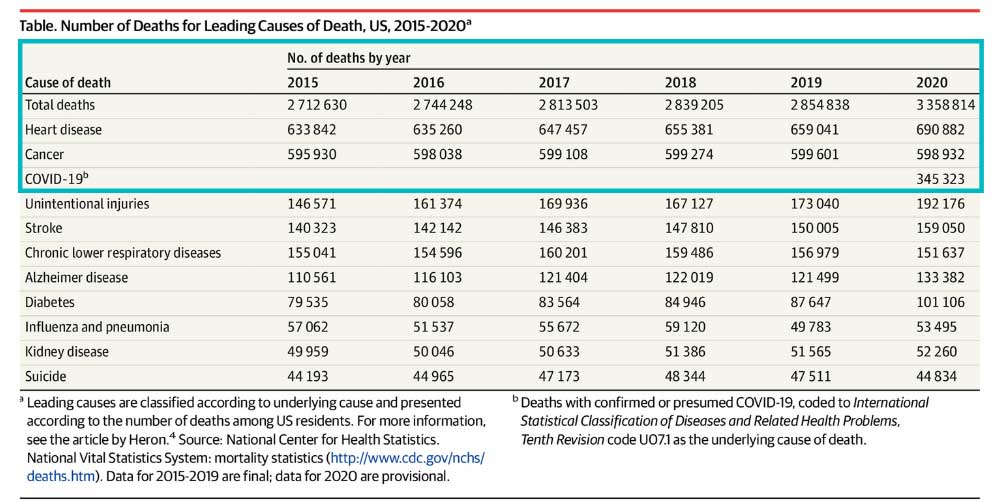
In 2020 heart disease killed twice as many people as COVID-19 in the United States.1 Some may find this surprising due to the lack of news coverage on heart disease. Historically heart disease has always been one of America’s most serious epidemics. It has been a leading cause of death since the turn of the 20th Century. Following World War II, the National Heart, Lung and Blood Institute began a long-term study known as the Framingham study to identify the cause of heart disease. The Framingham study is an enormous observational study in Framingham, Massachusetts. Researchers conducted physical examinations on participants every two years to study contributing factors to heart disease and are now on their 3rd generation of participants. The Framingham study identified many currently known risk factors, such as high blood pressure and high cholesterol. Researchers began developing medications to combat cholesterol levels once high cholesterol was identified as a significant risk factor. Some of our most exciting research at ENCORE Research Group is for new cholesterol-lowering medications such as Antisense Oligonucleotides (ASOs), Small Interfering RNA (siRNAs), and Adnectins. Antisense oligonucleotides (ASOs) are short, synthetic single-stranded fragments of RNA that can reduce, restore, or modify protein expression. ASOs have been designed specifically to target high levels of LDL (bad cholesterol) in the bloodstream in a different way than current medications. They are also being studied to reduce lipoprotein a [Lp (a) or “Lp little a”] in patients with elevated levels by targeting a building block of the Lp(a). Small interfering RNA (siRNAs) are another type of RNA therapy that is being used in clinical trials to reduce the risks of cardiovascular disease. Unlike ASOs which are single-stranded oligodeoxynucleotides, siRNAs are double-stranded RNA molecules. SiRNAs are used in the silencing of disease-causing genes for the treatment of atherosclerotic cardiovascular diseases. Adnectins are a class of drugs used to target proteins. Adnectins can be rapidly developed to bind proteins or other necessary targets. Currently, adnectins are being used in clinical trials to bind with a human protein called PCSK9. This binding blocks the interactions between PCSK9 and LDL (bad cholesterol) receptors. As a result, the levels of LDL cholesterol in the body are lowered. We are optimistic about these new technologies; they may give us the arsenal to fight back against heart disease. If you have high cholesterol levels that are not being adequately managed by your current medications, we may be able to help you get involved in a research study that may help get you back on track! As many of our readers know, most research studies offer access to cutting-edge therapies at no cost to patients. Call us to find out how you can get involved today! [1] CDC, https://www.cdc.gov/nchs/products/databriefs/db427.htm
5 Things to Know about Lp(a) Lipoprotein(a), or Lp(a), is an independent risk factor for atherosclerotic cardiovascular disease. Cardiovascular disease is the leading cause of death in both men and women in the US and globally . You may have heard of LDL cholesterol, or “bad cholesterol,” as a risk factor for heart disease, but Lp(a) can be just as dangerous. Lp(a) flies under the radar of many physicians. This is because the awareness of Lp(a) is still very low, very little is understood about the protein and the treatment options are limited. What is LP(a)? Lp(a), pronounced “LP little a,” is a protein that is attached to LDL cholesterol. It is composed of an LDL-like particle, but it has a second protein coiled around it. Recent studies have shown that people born with elevated Lp(a) can be two to four times as likely to have a heart attack or serious cardiac related risk. Lp(a) is present in 20% of the population. What differentiates LP(a) from other heart disease risk factors? LP(a) is so unique because it is a completely genetic risk factor. Meaning, having an elevated LP(a) is almost entirely determined by the genes you inherit. There is no evidence that a healthy lifestyle will lower your Lp(a). However, that does not mean those with high levels shouldn’t practice healthy habits. Reducing other risk factors that are determined by quality of health can still reduce the overall risk of heart disease. Another risk factor that sets LP(a) apart is that it is an independent risk factor. It has been linked to heart disease in younger adults who are otherwise healthy and have no prior cardiovascular risks. Elevated LP(a) has affected the lives of many who are otherwise healthy. For example, Tennis legend Arthur Ashe, who had his first heart attack at age 36. Bob Harper, a celebrity fitness trainer was also affected and nearly died of a heart attack at age 52. Who should be tested for Lp(a)? Studies show that there is a higher risk of a cardiovascular event if Lp(a) levels start to rise above 30 mg/dl. There is an even greater risk at levels 50 mg/dl and higher. There are an estimated one in seven people at or above this threshold. If you’ve had a cardiac event but your cholesterol levels are normal, or you have a family member with heart disease at an early age, have a cardiovascular event despite normal lipid levels, have a family history of Lp(a), or have aortic valvular disease at an early age then you should get tested for Lp(a). As mentioned, Lp(a) is a genetically mediated risk factor. “This means it runs in families,” Albert Lopez, MD, DO, FASPC, internal physician and lipid specialist in Jacksonville, FL says. “Those individuals that have it, you have a 50% chance of giving to your children.” Dr. Lopez believes there should be cascade screening, meaning asking family members if they have it and then getting tested. No FDA approved remedies for Lp(a) Currently there are no FDA approved remedies for elevated Lp(a). Statins, a widely known and used therapy that lowers LDL cholesterol does not reduce Lp(a) and has been shown to sometimes result in a slight increase. One therapy that has been shown to work is asphersis. This process filters a patient’s blood by circulating it through a machine and removing Lp(a) particles. However, this process is reserved for high-risk patients because it is extremely expensive, requires weekly visits and involves risks. After stopping apheresis, the Lp(a) levels begin to rise again. New Advancements in Science regarding Lp(a) Luckily, there are new drugs on the horizon that could potentially help those suffering from elevated Lp(a) levels. “What is exciting is that we are in totally nerd, sci-fi treatments now,” Dr. Lopez says. “We can actually stop your genes from making this protein by using a little snip that crinkles it up and doesnt let it read.” In other words, new studies are using gene silencing techniques to achieve a large and durable reduction of Lp(a). These therapies and medicines are still in clinical trials now. ENCORE Research group is conducting research studies for people with elevated Lp(a) in hopes to find a drug that will lower Lp(a) levels. It is up to the public to participate in these research studies to help those suffering from elevated Lp(a) levels.
For some patients, managing cholesterol creates a challenge. Statins are a safe and standard treatment but many people have very high levels of cholesterol that require more than statin drugs alone. Others cannot easily tolerate statins. Nonetheless, treating cholesterol saves lives and avoids heart attacks and strokes. Thankfully, the future is here with new breakthroughs that can change the way we maintain healthy cholesterol levels due to continued research and clinical trial participants. We outline some of these exciting technologies below. Antisense oligonucleotides (ASOs) are short, synthetic single stranded fragments of RNA that can reduce, restore or modify protein expression. ASOs have been designed specifically to target high levels of LDL (bad cholesterol) in the bloodstream in a different way than current medications. Firstly, ASOs targets the source of the disease resulting in a higher chance of success compared to therapies targeting downstream pathways. Secondly, ASOs are not metabolized by cytochrome P450 as most other drugs are. This significantly reduces the chance of one drug interacting with another drug in the body which could potentially cause more harm than good. Small interfering RNA (SiRNAs) are another type of RNA therapy that is being used in clinical trials to reduce the risks of cardiovascular disease. Unlike ASOs which are single-stranded oligodeoxynucleotides, siRNAs are double-stranded RNA molecules. SiRNAs are used in the silencing of disease-causing genes, in this case the genes involved in creating cardiovascular diseases, and it has made great progress. Adnectins are a class of drugs used to target proteins. Adnectins can be rapidly developed to bind proteins or other necessary targets. Currently, adnectins are being used in clinical trials to bind with a human protein called PCSK9. This binding blocks the interactions between PCSK9 and LDL (bad cholesterol) receptors. As a result, the levels of LDL cholesterol in the body are lowered. These technologies hold the potential to not only better manage cholesterol levels and thereby reducing heart attack and stroke risk, but many other conditions as well. Source: US National Library of Medicine
National Institutes of Health
What Are Triglycerides? Triglycerides are a type of fat (lipid) found in your blood. You get them in two ways – from the food you eat and from what your liver makes. Eating too many calories, especially from high carbohydrate foods, could lead to high triglycerides (hypertriglyceridemia), as could certain medications. High triglycerides could also be a sign of diabetes or thyroid problems, or be genetic. Almost 1 in 3 Americans have high triglycerides. When you have excess triglycerides, they are stored in the fat cells for later use. When they are needed, your body releases them as fatty acids, which fuel body movement, create heat, and provide energy for the body processes. A fasting blood test can tell where your triglyceride level falls. For good health, your triglyceride level should be less than 150 mg/dL. Borderline high levels are 150-199 mg/dL. High is 200-499 mg/dL. Very high is more than 500 mg/dL Diet and Lifestyle Changes to reduce High Triglycerides Consume less sugar and refined carbohydrates – limit white breads, white rice, white potatoes, sweetened beverages, sugary cereals, cakes and cookies. Instead choose whole grain breads, quinoa or wild rice, and fresh fruits and vegetables. Aim for 30 grams of fiber a day. Choose Healthy fats – use unsaturated fats such as olive and avocado oils. Eat fish, poultry, less red meat, and enjoy some meatless meals. Limit your intake of alcohol – for some people drinking even a little bit can have a big effect on triglycerides. One of the best ways to lower triglycerides is with regular exercise. Aim for an average of 40 minutes of moderate to high intensity exercise on 3 to 4 days a week. Taking a brisk walk every day works for many people. When Healthy Lifestyle Changes Are Not Enough Your doctor may recommend medication to help lower your high triglycerides, such as nicotinic acid (niacin), fibrates, omega-3-fatty acids (fish oil) or statins. There are also some new medications being developed that may not only lower your triglycerides, but reduce your risk of heart disease overall. Many of our research sites are participating in these important clinical trials. We invite you to contact one of our sites near you to see if you could benefit from one of these programs. Lori Alexander, MSHS, RDN, CCRC, CLS, FNLA Director, ENCORE Lipid Center of Excellence
Cholesterol has earned a bad reputation over the years. However, it is required by every part of your body for day to day functions. In fact, cholesterol is so important to daily function, that every cell in the body can make cholesterol from basic materials, except your eyelashes! So how do you reconcile these two completely different ideas? The cholesterol that circulates in your blood stream is the extra stuff that your body is trying to get rid of. This extra cholesterol is what can cause damage to arteries, heart disease, and increase your risk for stroke.
So, what is cholesterol? It is a type of waxy, fat-like substance, also called a lipid. Since cholesterol is a fat, it can’t travel alone in the bloodstream. It would end up as useless globs (imagine bacon fat floating in a pot of water). To get around this problem, the body packages cholesterol and other lipids into minuscule protein-covered particles that mix easily with blood. These tiny particles, called lipoproteins (lipid plus protein), move cholesterol and other fats throughout the body.1
LDL (low-density lipoprotein) is considered the “bad”, unhealthy cholesterol that can build up in the arteries and form deposits called plaques.
HDL (high-density lipoprotein) is the “good”, healthy kind of cholesterol that transports excess LDL cholesterol to the liver to be removed from the body.
PCSK9 is a protein in our body that regulates the circulating levels of LDL “bad” cholesterol. Decreasing the PCSK9 proteins in the body will reduce LDL levels and reduce the risk of heart attack and stroke.
There are currently two FDA approved medications that have been very successful in blocking the PCSK9 protein once it has been made. They are Repatha and Praluent. However, the medications are expensive and not approved for all patients under their insurance.
Scientists believe it would be even more powerful to prevent the PCSK9 protein from being made in the first place. Currently being studied are a new class of molecules called antisense oligonucleotides (ASO). ASOs are pieces of DNA that short-circuit the production of PCSK9, resulting in reduced LDL levels and associated risks.
When you participate in a clinical research study, you gain access to these types of cutting-edge therapies at no cost and before the general population. Contact us to schedule a free consultation to see if you qualify for one of our clinical research studies. If you qualify for one of our clinical trials, your health will be closely monitored by our team of expert medical professionals throughout the trial.
Reference:
- https://www.health.harvard.edu/heart-health/how-its-made-cholesterol-production-in-your-body
You may know that having normal cholesterol levels in your blood are important for helping prevent heart attacks and strokes. But you may not know that there is another factor in your blood work that can be just as important! It’s a blood test you can request, and the result will tell you if it’s important for you.
Lipoprotein(a) is a particle in your blood which carries cholesterol, fats, and proteins. The amount your body makes is inherited and determined by the time you are born. It does not change very much during your life and is not affected by diet or exercise.
Lipoprotein(a) is also known as Lp(a), L-p-a, Lipoprotein-little-a, and L-p-little-a. Some cholesterol and Lp(a) in your blood is normal. A high level of LDL, the bad cholesterol, increases your risk for heart attack or stroke. High levels of Lp(a) also increase your risk, even if your cholesterol numbers are normal! About 20 percent of people, or 1 in 5, have high levels of Lp(a). This blood test is not done as part of your usual blood testing but can be requested.
Here are some reasons an Lp(a) blood test may be important for you:
- Having high levels of LDL, even while taking medicine to treat it.
- You or a family member have had a heart attack or stroke at an early age, men younger than 55 years old and women less than 65 years old.
- You or a family member developed high blood pressure at an early age.
- A family member has high Lp(a). If an adult has high Lp(a), their children have a 1 in 2 chance of inheriting it.
- Having FH, Familial Hypercholesterolemia, an inherited condition of very high cholesterol levels.
Get tested, ask your nurse or doctor if you have questions.
- Join a clinical trial. Jacksonville Center for Clinical Research currently has an enrolling study, call 904-730-0166 for more information. If you are eligible for this study, Lp(a) testing is provided at no cost to you.
- Ask your Doctor to order the lab test. Several labs perform it:
- Cleveland Heart Lab / Quest
- HDL labs
- Boston Heart Diagnostics
Be aware of your personal risks.
Reach healthy goals for your cholesterol results with dietary choices, exercise, and medication if needed.
Stay healthy, stay active, exercise, eat naturally, have fun, love, laugh!
Written by: Julia Baker, RN, adapted from a presentation by Albert Lopez, MD
Heart disease currently accounts for 1 in 4 deaths in the United States.[1] However due to new research breakthroughs there are now treatments available that may finally give us the means to fight back against heart disease. Historically heart disease has always been one of America’s most serious epidemics. It has been a leading cause of death since the turn of the 20th Century. Following World War II the National Heart, Lung and Blood Institute began a long term study known as the Framingham study to identify the cause of heart disease.
The Framingham study is an enormous observational study in the town of Framingham, Massachusetts. Researchers conducted physical examinations on participants every two years to study contributing factors to heart disease and are now on their 3rd generation of participants. The Framingham study identified many of the currently known risk factors such as high blood pressure and high cholesterol. Once high cholesterol was identified as a major risk factor, researchers began developing medications to combat cholesterol levels. Some of our most exciting research at Encore Research Group is for these new cholesterol lowering medications such as PCSK9 inhibitors.
PCSK9 inhibitors are an amazing class of drugs that allow us to lower LDL or “bad cholesterol” to previously unachievable levels. These drugs are usually injectable and have many advantages over traditional statin drugs. One such advantage is we are not seeing the muscle cramping associated with statin therapy. This is truly a breakthrough.
How low is too low? It is a question that researchers are actively addressing. So far we have not seen complications or health risks as a result of very low LDL. Some large studies that Encore Research has participated in will be releasing their results within the next year to more definitively answer this question. For now, there are many patients that have cholesterol levels that are difficult to budge but may respond to these new therapies.
Currently we are studying the effects of PCSK9 drugs in high risk populations such as diabetics. If you have high cholesterol levels that are not being adequately managed by your current medications, we may be able to help you get involved in a research study that may help get you back on track! As many of our readers know, most research studies offer access to medication at no cost to patients. Call us to find out how you can get involved today!
[1] CDC, NCHS. Underlying Cause of Death 1999-2013 on CDC WONDER Online Database, released 2015. Data are from the Multiple Cause of Death Files, 1999-2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed Feb. 3, 2015.
If you have high cholesterol you may dread going to your doctor, especially if they are going to complete a cholesterol blood test. You know they prescribed a statin, but the muscle cramping you experience after taking it just isn’t worth it. How do you tell your doctor that the medication they prescribed just isn’t working for you? You are not alone, and there are options available for you.
We all know that having excess cholesterol in our blood is a bad thing, but why is it so bad? High cholesterol has often been called ‘The Silent Killer’. In fact, according to the CDC heart disease is responsible for 1 in 4 American deaths every year.[1] High cholesterol is known to cause plaque formation in arteries, constricting blood flow to vital organs in your body. Even worse, cholesterol plaques can become dislodged from the walls of the arteries potentially causing blood clots. Both heart attacks and strokes can be caused by plaques reaching the heart or brain respectively. If lifestyle changes such as a good diet and exercise can’t bring down your cholesterol numbers, you may need a medication. The most common cholesterol lowering medications to date are statins such as Crestor, Lipitor, or Zocor. These medicines have been life saving for many people that can tolerate them. However, some people are intolerant to statins and will experience side effects such as painful muscle cramps, inflammation and more.
If you are allergic to or can’t handle statins what can you do? It is crucial to keep your cholesterol levels down, lowering your risk for a heart attack and stroke. You may try one of the medications already on the market for people with statin intolerance such as Zetia, Juxtapid and Repatha. However, each of these drugs have their own risks. Zetia can cause symptoms similar to those caused by statins. Juxtapid, a newer medication, has been found to significantly reduce LDL bad cholesterol by 40-50%. Sadly, it also caused diarrhea, nausea, vomiting or abdominal pain in 28% of patients.[2] In 2015 the FDA approved Repatha, a new class of drug called a PCSK9 inhibitor that is very successful in lowering LDL. Unfortunately, due to the cost of development and production the annual cost is around $14,000 dollars making it unaffordable for most people.
If you’ve had trouble taking statins in the past you may be asking “what do I do now”? Many of our participants are looking for alternative treatments or want to be part of cutting edge research. I encourage you to check out the cholesterol research studies we are conducting at many of our research centers. You may qualify for a new oral medication or to receive PCSK9 in an upcoming study! The medicines being researched for people who cannot take statins may significantly alter the future of cardiovascular disease. We need your help to bring these new medications to market!
References:
[1] “Heart Disease Fact Sheet.” Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 16 June 2016. Web. 27 Apr. 2017.
[2] Orrange, Sharon, MD. “Finally, a Non-Statin Cholesterol Medication That Works: Introducing Juxtapid.” The GoodRx Prescription Savings Blog. N.p., 06 June 2014. Web. 27 Apr. 2017.
Familial Chylomicronemia Syndrome (FCS) is a rare genetic disorder characterized by the build-up of chylomicrons, which are rich in triglycerides, a type of fat in your blood. The human body naturally produces an enzyme to eliminate the chylomicrons called lipoprotein lipase (LPL). However, people with FCS either do not have the LPL enzyme or it does not function properly. Due to the buildup of chylomicrons and very high triglycerides, they live at risk of severe recurrent abdominal pain and potentially fatal pancreatitis, as well as decreased quality of life in many areas.
To this day, there is no treatment for FCS; the current medications used for lowering triglyceride levels do not work for people with FCS since they are designed to work on the LPL enzyme. Currently, the only option is to follow an ultra-low or no fat diet, which is very difficult to maintain long term. People with FCS also must avoid alcohol, sweets, and simple carbohydrates due to their effect on raising triglycerides.
Lori Alexander is Director of the Lipid Center of Excellence at Jacksonville Center for Clinical Research. She is a Registered Dietitian Nutritionist and board member of the National Lipid Association (NLA) and the Foundation of the National Lipid Association. Lori has been coordinating clinical trials with Akcea, one involving a new potential treatment for people with FCS called volanesorsen (Waylivra).
Recently, Lori was asked to testify on behalf of people with FCS. On May 10, 2018 she traveled to the Food and Drug Administration (FDA) Headquarters in Bethesda, MD to testify in front of FDA Endocrinologic and Metabolic Drugs Advisory Committee. She was accompanied by Akcea scientists who shared clinical trial data, and some people diagnosed with FCS who got to share their stories, some of whom saw great relief from volanesorsen during the clinical trials. During her testimony she encouraged the approval of volanesorsen (Waylivra), “Treatment with volanesorsen offers hope to these people, many who have been struggling for years without medical therapy”. 1
After hearing from the Akcea scientists, the people with FCS and Lori’s testimony, the advisory committee voted 12-8 in favor of recommending volanesorsen’s approval to the FDA! The FDA has set a date for the completion of its review on August 30, 2018. 2
References:
- https://www.lipid.org/nla/alexander-testifies-front-fda-advisory-committee-behalf-fcs-patients
- http://www.raredr.com/news/fda-votes-in-favor-of-fcs-drug
Photo Credit: National Lipid Association